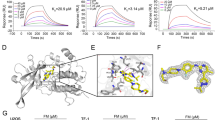
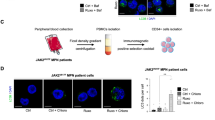
Abstract
Mutations in the Janus Kinase 2 (JAK2) gene resulting in constitutive kinase activation represent the most common genetic event in myeloproliferative neoplasms (MPN), a group of diseases involving overproduction of one or more kinds of blood cells, including red cells, white cells, and platelets. JAK2 kinase inhibitors, such as ruxolitinib, provide clinical benefit, but inhibition of wild-type (wt) JAK2 limits their clinical utility due to toxicity to normal cells, and small molecule inhibition of mutated JAK2 kinase activity can lead to drug resistance. Here, we present a strategy to target mutated JAK2 for degradation, using the cell’s intracellular degradation machinery, while sparing non-mutated JAK2. We employed a chemical genetics screen, followed by extensive selectivity profiling and genetic studies, to identify the deubiquitinase (DUB), JOSD1, as a novel regulator of mutant JAK2. JOSD1 interacts with and stabilizes JAK2-V617F, and inactivation of the DUB leads to JAK2-V617F protein degradation by increasing its ubiquitination levels, thereby shortening its protein half-life. Moreover, targeting of JOSD1 leads to the death of JAK2-V617F-positive primary acute myeloid leukemia (AML) cells. These studies provide a novel therapeutic approach to achieving selective targeting of mutated JAK2 signaling in MPN.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Gäbler K, Behrmann I, Haan C. JAK2 mutants (e.g., JAK2V617F) and their importance as drug targets in myeloproliferative neoplasms. JAK-STAT. 2013;2:e25025.
James C, Ugo V, Le Couedic JP, Staerk J, Delhommeau F, Lacout C, et al. A unique clonal JAK2 mutation leading to constitutive signalling causes polycythaemia vera. Nature 2005;434:1144–8.
Baxter EJ, Scott LM, Campbell PJ, East C, Fourouclas N, Swanton S, et al. Acquired mutation of the tyrosine kinase JAK2 in human myeloproliferative disorders. Lancet 2005;365:1054–61.
Kralovics R, Passamonti F, Buser AS, Teo SS, Tiedt R, Passweg JR, et al. A gain-of-function mutation of JAK2 in myeloproliferative disorders. N Engl J Med. 2005;352:1779–90.
Levine RL, Wadleigh M, Cools J, Ebert BL, Wernig G, Huntly BJ, et al. Activating mutation in the tyrosine kinase JAK2 in polycythemia vera, essential thrombocythemia, and myeloid metaplasia with myelofibrosis. Cancer Cell. 2005;7:387–97.
Campbell PJ, Green AR. The myeloproliferative disorders. N Engl J Med. 2006;355:2452–66.
Pikman Y, Lee BH, Mercher T, McDowell E, Ebert BL, Gozo M, et al. MPLW515L is a novel somatic activating mutation in myelofibrosis with myeloid metaplasia. PLoS Med. 2006;3:e270.
Rumi E, Pietra D, Guglielmelli P, Bordoni R, Casetti I, Milanesi C, et al. Acquired copy-neutral loss of heterozygosity of chromosome 1p as a molecular event associated with marrow fibrosis in MPL-mutated myeloproliferative neoplasms. Blood 2013;121:4388–95.
Klampfl T, Gisslinger H, Harutyunyan AS, Nivarthi H, Rumi E, Milosevic JD, et al. Somatic mutations of calreticulin in myeloproliferative neoplasms. N Engl J Med. 2013;369:2379–90.
Imai M, Araki M, Komatsu N. Somatic mutations of calreticulin in myeloproliferative neoplasms. Int J Hematol. 2017;105:743–7.
Araki M, Yang Y, Masubuchi N, Hironaka Y, Takei H, Morishita S, et al. Activation of the thrombopoietin receptor by mutant calreticulin in CALR-mutant myeloproliferative neoplasms. Blood 2016;127:1307–16.
Quintas-Cardama A, Vaddi K, Liu P, Manshouri T, Li J, Scherle PA, et al. Preclinical characterization of the selective JAK1/2 inhibitor INCB018424: therapeutic implications for the treatment of myeloproliferative neoplasms. Blood 2010;115:3109–17.
Verstovsek S, Kantarjian H, Mesa RA, Pardanani AD, Cortes-Franco J, Thomas DA, et al. Safety and efficacy of INCB018424, a JAK1 and JAK2 inhibitor, in myelofibrosis. N Engl J Med. 2010;363:1117–27.
Vainchenker W, Leroy E, Gilles L, Marty C, Plo I, Constantinescu SN. JAK inhibitors for the treatment of myeloproliferative neoplasms and other disorders. F1000Res. 2018;7:82. -
Meyer SC, Levine RL. Molecular pathways: molecular basis for sensitivity and resistance to JAK kinase inhibitors. Clin Cancer Res. 2014;20:2051–9.
Koppikar P, Bhagwat N, Kilpivaara O, Manshouri T, Adli M, Hricik T, et al. Heterodimeric JAK-STAT activation as a mechanism of persistence to JAK2 inhibitor therapy. Nature 2012;489:155–9.
Andraos R, Qian Z, Bonenfant D, Rubert J, Vangrevelinghe E, Scheufler C, et al. Modulation of activation-loop phosphorylation by JAK inhibitors is binding mode dependent. Cancer Discov. 2012;2:512.
Scheffner M, Nuber U, Huibregtse JM. Protein ubiquitination involving an E1–E2–E3 enzyme ubiquitin thioester cascade. Nature 1995;373:81–3.
Ungureanu D, Saharinen P, Junttila I, Hilton DJ, Silvennoinen O. Regulation of Jak2 through the ubiquitin-proteasome pathway involves phosphorylation of Jak2 on Y1007 and Interaction with SOCS-1. Mol Cell Biol. 2002;22:3316.
Lv K, Jiang J, Donaghy R, Riling CR, Cheng Y, Chandra V, et al. CBL family E3 ubiquitin ligases control JAK2 ubiquitination and stability in hematopoietic stem cells and myeloid malignancies. Genes Dev. 2017;31:1007–23.
Chou DH-C, Vetere A, Choudhary A, Scully SS, Schenone M, Tang A, et al. Kinase-independent small-molecule inhibition of JAK-STAT signaling. J Am Chem Soc. 2015;137:7929–34.
Kapuria V, Peterson LF, Fang D, Bornmann WG, Talpaz M, Donato NJ. Deubiquitinase inhibition by small-molecule WP1130 triggers aggresome formation and tumor cell apoptosis. Cancer Res. 2010;70:9265.
Kapuria V, Levitzki A, Bornmann WG, Maxwell D, Priebe W, Sorenson RJ, et al. A novel small molecule deubiquitinase inhibitor blocks Jak2 signaling through Jak2 ubiquitination. Cell Signal. 2011;23:2076–85.
Weisberg EL, Schauer NJ, Yang J, Lamberto I, Doherty L, Bhatt S, et al. Inhibition of USP10 induces degradation of oncogenic FLT3. Nat Chem Biol. 2017;13:1207–15.
Wernig G, Gonneville JR, Crowley BJ, Rodrigues MS, Reddy MM, Hudon HE, et al. The Jak2V617F oncogene associated with myeloproliferative diseases requires a functional FERM domain for transformation and for expression of the Myc and Pim proto-oncogenes. Blood 2008;111:3751–9.
Lamberto I, Liu X, Seo HS, Schauer NJ, Iacob RE, Hu W. et al. Structure-guided development of a potent and selective non-covalent active-site inhibitor of USP7. Cell Chem Biol. 2017;24:1490–500. e11.
Yang J, Meng C, Weisberg E, Case A, Lamberto I, Magin RS, et al. Inhibition of the deubiquitinase USP10 induces degradation of SYK. Br J of Cancer. 2020;122:1175–84.
WO2016046530.compound 120.
Altun M, Kramer Holger B, Willems Lianne I, McDermott Jeffrey L, Leach Craig A, Goldenberg Seth J, et al. Activity-based chemical proteomics accelerates inhibitor development for deubiquitylating enzymes. Chem Biol. 2011;18:1401–12.
Kluge AF, Lagu BR, Maiti P, Jaleel M, Webb M, Malhotra J, et al. Novel highly selective inhibitors of ubiquitin specific protease 30 (USP30) accelerate mitophagy. Bioorg Med Chem Lett. 2018;28:2655–9.
Schauer NJ, Magin RS, Liu X, Doherty LM, Buhrlage SJ. Advances in discovering deubiquitinating enzyme (DUB) inhibitors. J Med Chem. 2020;63:2731–50.
Wu X, Luo Q, Zhao P, Chang W, Wang Y, Shu T, et al. JOSD1 inhibits mitochondrial apoptotic signalling to drive acquired chemoresistance in gynaecological cancer by stabilizing MCL1. Cell Death Differ. 2020;27:55–70.
Wang X, Zhang L, Zhang Y, Zhao P, Qian L, Yuan Y, et al. JOSD1 negatively regulates Type-I interferon antiviral activity by deubiquitinating and stabilizing SOCS1. Viral Immunol. 2017;30:342–9.
Wernig G, Mercher T, Okabe R, Levine RL, Lee BH, Gilliland DG. Expression of Jak2V617F causes a polycythemia vera-like disease with associated myelofibrosis in a murine bone marrow transplant model. Blood 2006;107:4274–81.
Harrison C, Kiladjian JJ, Al-Ali HK, Gisslinger H, Waltzman R, Stalbovskaya V, et al. JAK inhibition with ruxolitinib versus best available therapy for myelofibrosis. N Engl J Med. 2012;366:787–98.
Verstovsek S, Mesa RA, Gotlib J, Levy RS, Gupta V, DiPersio JF, et al. A double-blind, placebo-controlled trial of ruxolitinib for myelofibrosis. N Engl J Med. 2012;366:799–807.
Eghtedar A, Verstovsek S, Estrov Z, Burger J, Cortes J, Bivins C, et al. Phase 2 study of the JAK kinase inhibitor ruxolitinib in patients with refractory leukemias, including postmyeloproliferative neoplasm acute myeloid leukemia. Blood 2012;119:4614–8.
Ajayi S, Becker H, Reinhardt H, Engelhardt M, Zeiser R, von Bubnoff N, et al. Ruxolitinib. Recent Results Cancer Res. 2018;212:119–32.
Kong X, Sun H, Pan P, Li D, Zhu F, Chang S, et al. How does the L884P mutation confer resistance to Type-II inhibitors of JAK2 kinase: a comprehensive molecular modeling study. Sci Rep. 2017;7:9088.
Mohapatra B, Ahmad G, Nadeau S, Zutshi N, An W, Scheffe S, et al. Protein tyrosine kinase regulation by ubiquitination: critical roles of Cbl-family ubiquitin ligases. Biochim Biophys Acta. 2013;1833:122–39.
Acknowledgements
We thank Dr. Nathanael Gray for their generous provision of reagents and technical support, and Dr. Lucia Cabal-Hierro at Dana-Farber Cancer Institute—Harvard Medical School for valuable guidance for the CRISPR-CAS9 KO assay. Our work was founded by Leukemia & Lymphoma Society’s New Idea Award, Claudia Adams Barr Award, MPN Research Foundation, Gabrielle’s Angel Foundation and NIH R01 Foundation CA211681.
Author information
Authors and Affiliations
Contributions
JY, EW and XL conceptualized, designed and performed the studies. JY, EW and XL carried out data analyses and wrote the manuscript. RM purified JOSD1 protein and performed biochemical assay for compounds testing. WC and NS performed ABPP assay for protein profiling. BH assisted with compounds designed and synthesized. SZ, IL, LD and LC-H assisted with shRNA KD/KO studies. CM helped with cell culturing and western blotting. MS provided valuable scientific feedback and guidance. JW and NG provided JAK1/JAK2 inhibitors. JAM carried out quantitative mass spectrometry. JG conceptualized and designed the studies. SB conceptualized and designed the studies, carried out data analyses and wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Yang, J., Weisberg, E.L., Liu, X. et al. Small molecule inhibition of deubiquitinating enzyme JOSD1 as a novel targeted therapy for leukemias with mutant JAK2. Leukemia 36, 210–220 (2022). https://doi.org/10.1038/s41375-021-01336-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41375-021-01336-9
This article is cited by
-
Pharmaceutical targeting of OTUB2 sensitizes tumors to cytotoxic T cells via degradation of PD-L1
Nature Communications (2024)
-
Inhibition of the deubiquitinating enzyme USP47 as a novel targeted therapy for hematologic malignancies expressing mutant EZH2
Leukemia (2022)
-
Structural basis for specific inhibition of the deubiquitinase UCHL1
Nature Communications (2022)
-
Development and validation of a transcriptomic signature-based model as the predictive, preventive, and personalized medical strategy for preterm birth within 7 days in threatened preterm labor women
EPMA Journal (2022)