Abstract
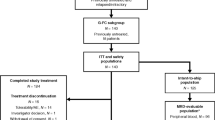
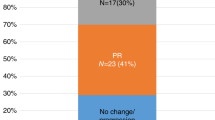
Chemoimmunotherapy with combined fludarabine, cyclophosphamide and rituximab (FCR) has been an effective treatment for patients with chronic lymphocytic leukemia (CLL). We initiated a phase II trial for previously untreated patients with CLL with mutated IGHV and absence of del(17p)/TP53 mutation. Patients received ibrutinib, fludarabine, cyclophosphamide, and obinutuzumab (iFCG) for three cycles. Patients who achieved complete remission (CR)/CR with incomplete count recvoery (CRi) with marrow undetectable measurable residual disease (U-MRD) received additional nine cycles of ibrutinib with three cycles of obinutuzumab; all others received nine additional cycles of ibrutinib and obinutuzumab. Patients in marrow U-MRD remission after cycle 12 discontinued all treatment, including ibrutinib. Forty-five patients were treated. The median follow-up is 41.3 months. Among the total 45 treated patients, after three cycles, 38% achieved CR/CRi and 87% achieved marrow U-MRD. After cycle 12, the corresponding numbers were 67% and 91%, respectively. Overall, 44/45 (98%) patients achieved marrow U-MRD as best response. No patient had CLL progression. The 3-year progression-free survival (PFS) and overall survival (OS) were 98% and 98%, respectively. Per trial design, all patients who completed cycle 12 discontinued ibrutinib, providing for a time-limited therapy. Grade 3–4 neutropenia and thrombocytopenia occurred in 58% and 40% patients, respectively. The iFCG regimen with only 3 cycles of chemotherapy is an effective, time-limited regimen for patients with CLL with mutated IGHV and without del(17p)/TP53 mutation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Hallek M, Shanafelt TD, Eichhorst B. Chronic lymphocytic leukaemia. Lancet. 2018;391:1524–37.
Keating MJ, O’Brien S, Albitar M, Lerner S, Plunkett W, Giles F, et al. Early results of a chemoimmunotherapy regimen of fludarabine, cyclophosphamide, and rituximab as initial therapy for chronic lymphocytic leukemia. J Clin Oncol. 2005;23:4079–88.
Eichhorst B, Fink AM, Bahlo J, Busch R, Kovacs G, Maurer C, et al. First-line chemoimmunotherapy with bendamustine and rituximab versus fludarabine, cyclophosphamide, and rituximab in patients with advanced chronic lymphocytic leukaemia (CLL10): an international, open-label, randomised, phase 3, non-inferiority trial. Lancet Oncol. 2016;17:928–42.
Benjamini O, Jain P, Trinh L, Qiao W, Strom SS, Lerner S, et al. Second cancers in patients with chronic lymphocytic leukemia who received frontline fludarabine, cyclophosphamide and rituximab therapy: distribution and clinical outcomes. Leuk Lymphoma. 2015;56:1643–50.
Fischer K, Bahlo J, Fink AM, Goede V, Herling CD, Cramer P, et al. Long-term remissions after FCR chemoimmunotherapy in previously untreated patients with CLL: updated results of the CLL8 trial. Blood. 2016;127:208–15.
Kutsch N, Bahlo J, Robrecht S, Franklin J, Zhang C, Maurer C, et al. Long term follow-up data and health-related quality of life in frontline therapy of fit patients treated with FCR versus BR (CLL10 trial of the GCLLSG). Hemasphere. 2020;4:e336.
Thompson PA, Tam CS, O’Brien SM, Wierda WG, Stingo F, Plunkett W, et al. Fludarabine, cyclophosphamide, and rituximab treatment achieves long-term disease-free survival in IGHV-mutated chronic lymphocytic leukemia. Blood. 2016;127:303–9.
Rossi D, Terzi-di-Bergamo L, De Paoli L, Cerri M, Ghilardi G, Chiarenza A, et al. Molecular prediction of durable remission after first-line fludarabine-cyclophosphamide-rituximab in chronic lymphocytic leukemia. Blood. 2015;126:1921–4.
Byrd JC, Brown JR, O’Brien S, Barrientos JC, Kay NE, Reddy NM, et al. Ibrutinib versus ofatumumab in previously treated chronic lymphoid leukemia. N Engl J Med. 2014;371:213–23.
Burger JA, Tedeschi A, Barr PM, Robak T, Owen C, Ghia P, et al. Ibrutinib as initial therapy for patients with chronic lymphocytic leukemia. N Engl J Med. 2015;373:2425–37.
Shanafelt TD, Wang XV, Kay NE, Hanson CA, O’Brien S, Barrientos J, et al. Ibrutinib-rituximab or chemoimmunotherapy for chronic lymphocytic leukemia. N Engl J Med. 2019;381:432–43.
Tallman MS, Litzow M, Stone RM, Erba HP, Little RF, Mullane MP, et al. Ibrutinib and rituximab provides superior clinical outcome compared to FCR in younger patients with chronic lymphocytic leukemia (CLL): extended follow-up from the E1912 trial. Blood. 2019;134 (Suppl 1):33.
Bottcher S, Ritgen M, Fischer K, Stilgenbauer S, Busch RM, Fingerle-Rowson G, et al. Minimal residual disease quantification is an independent predictor of progression-free and overall survival in chronic lymphocytic leukemia: a multivariate analysis from the randomized GCLLSG CLL8 trial. J Clin Oncol. 2012;30:980–8.
Dimier N, Delmar P, Ward C, Morariu-Zamfir R, Fingerle-Rowson G, Bahlo J, et al. A model for predicting effect of treatment on progression-free survival using MRD as a surrogate end point in CLL. Blood. 2018;131:955–62.
Chanan-Khan A, Cramer P, Demirkan F, Fraser G, Silva RS, Grosicki S, et al. Ibrutinib combined with bendamustine and rituximab compared with placebo, bendamustine, and rituximab for previously treated chronic lymphocytic leukaemia or small lymphocytic lymphoma (HELIOS): a randomised, double-blind, phase 3 study. Lancet Oncol. 2016;17:200–11.
Goede V, Fischer K, Busch R, Engelke A, Eichhorst B, Wendtner CM, et al. Obinutuzumab plus chlorambucil in patients with CLL and coexisting conditions. N Engl J Med. 2014;370:1101–10.
Goede V, Fischer K, Dyer MJ, Muller L, Smolej L, Bernardo M, et al. Overall survival benefit of obinutuzumab over rituximab when combined with chlorambucil in patients with chronic lymphocytic leukemia and comorbidities: final survival analysis of the CLL11 study. Eur Hematol Assoc Annu Meet. 2018;S151.
Brown JR, O’Brien S, Kingsley CD, Eradat H, Pagel JM, Lymp J, et al. Obinutuzumab plus fludarabine/cyclophosphamide or bendamustine in the initial therapy of CLL patients: the phase 1b GALTON trial. Blood. 2015;125:2779–85.
Brown JR, O’Brien S, Kingsley CD, Eradat H, Pagel JM, Hirata J, et al. Durable remissions with obinutuzumab-based chemoimmunotherapy: long-term follow-up of the phase 1b GALTON trial in CLL. Blood. 2019;133:990–2.
Bosch F, Cantin G, Cortelezzi A, Knauf W, Tiab M, Turgut M, et al. Obinutuzumab plus fludarabine and cyclophosphamide in previously untreated, fit patients with chronic lymphocytic leukemia: a subgroup analysis of the GREEN study. Leukemia. 2020;34:441–50.
Hallek M, Cheson BD, Catovsky D, Caligaris-Cappio F, Dighiero G, Dohner H, et al. Guidelines for the diagnosis and treatment of chronic lymphocytic leukemia: a report from the International Workshop on Chronic Lymphocytic Leukemia updating the National Cancer Institute-Working Group 1996 guidelines. Blood. 2008;111:5446–56.
Rawstron AC, Villamor N, Ritgen M, Bottcher S, Ghia P, Zehnder JL, et al. International standardized approach for flow cytometric residual disease monitoring in chronic lymphocytic leukaemia. Leukemia. 2007;21:956–64.
Carlson CS, Emerson RO, Sherwood AM, Desmarais C, Chung MW, Parsons JM, et al. Using synthetic templates to design an unbiased multiplex PCR assay. Nat Commun. 2013;4:2680.
Strati P, Keating MJ, O’Brien SM, Burger J, Ferrajoli A, Jain N, et al. Eradication of bone marrow minimal residual disease may prompt early treatment discontinuation in CLL. Blood. 2014;123:3727–32.
Koyama T, Chen H. Proper inference from Simon’s two-stage designs. Stat Med. 2008;27:3145–54.
Jain N. Selecting frontline therapy for CLL in 2018. Hematol Am Soc Hematol Educ Progr. 2018;2018:242–7.
Foon KA, Boyiadzis M, Land SR, Marks S, Raptis A, Pietragallo L, et al. Chemoimmunotherapy with low-dose fludarabine and cyclophosphamide and high dose rituximab in previously untreated patients with chronic lymphocytic leukemia. J Clin Oncol. 2009;27:498–503.
Thompson PA, Srivastava J, Peterson C, Strati P, Jorgensen JL, Hether T, et al. Minimal residual disease undetectable by next-generation sequencing predicts improved outcome in CLL after chemoimmunotherapy. Blood. 2019;134:1951–9.
Michallet AS, Dilhuydy MS, Subtil F, Rouille V, Mahe B, Laribi K, et al. Obinutuzumab and ibrutinib induction therapy followed by a minimal residual disease-driven strategy in patients with chronic lymphocytic leukaemia (ICLL07 FILO): a single-arm, multicentre, phase 2 trial. Lancet Haematol. 2019;6:e470–9.
Davids MS, Brander DM, Kim HT, Tyekucheva S, Bsat J, Savell A, et al. Ibrutinib plus fludarabine, cyclophosphamide, and rituximab as initial treatment for younger patients with chronic lymphocytic leukaemia: a single-arm, multicentre, phase 2 trial. Lancet Haematol. 2019;6:e419–28.
Sharman JP, Egyed M, Jurczak W, Skarbnik A, Pagel JM, Flinn IW, et al. Acalabrutinib with or without obinutuzumab versus chlorambucil and obinutuzmab for treatment-naive chronic lymphocytic leukaemia (ELEVATE TN): a randomised, controlled, phase 3 trial. Lancet. 2020;395:1278–91.
Fischer K, Al-Sawaf O, Bahlo J, Fink AM, Tandon M, Dixon M, et al. Venetoclax and obinutuzumab in patients with CLL and coexisting conditions. N Engl J Med. 2019;380:2225–36.
Jain N, Keating M, Thompson P, Ferrajoli A, Burger J, Borthakur G, et al. Ibrutinib and venetoclax for first-line treatment of CLL. N Engl J Med. 2019;380:2095–103.
Jain N, Keating MJ, Thompson PA, Ferrajoli A, Burger JA, Borthakur G, et al. Combined ibrutinib and venetoclax for first-line treatment for patients with chronic lymphocytic leukemia (CLL): focus on MRD results. Blood. 2020;136(Suppl 1):42–43.
Wierda WG, Tam CS, Allan JN, Siddiqi T, Kipps TJ, Tedeschi A, et al. Ibrutinib (Ibr) plus venetoclax (Ven) for first-line treatment of chronic lymphocytic leukemia (CLL)/small lymphocytic lymphoma (SLL): 1-year disease-free survival (DFS) results from the MRD cohort of the phase 2 CAPTIVATE study. Blood. 2020;136(Supple 1):16–17.
Acknowledgements
Supported by Pharmacyclics LLC, an AbbVie Company and Genentech. This research is supported in part by the MD Anderson Cancer Center CLL Moon Shot program and MD Anderson Cancer Center Support Grant P30 CA016672. We thank the patients who participated in this trial and their families, the MDACC IND office for their oversight of the study, and the referring physicians. We thank the entire clinical and research staff at the Department of Leukemia, MDACC.
Funding
The study was supported in part with funding and drug supply (ibrutinib, obinutuzumab) by Pharmacyclics and Genentech. The pharmaceutical sponsors approved the study design and reviewed the paper, but had no role in the collection or analyses of the data.
Author information
Authors and Affiliations
Contributions
NJ, PT, JB, AF, ZE, HK, SOB, MK, and WW designed the study. NJ, PT, JB, AF, KT, ZE, GB, PB, TK, NP, KS, MK, EJ, HK, SOB, MK, and WW enrolled and managed patients on the trial. NG read the radiology reports for the trial. XW was the trial statistician. RKS and KP performed genomic sequencing. WW, JJ, and SW performed flow cytometry for MRD. WL and AA were study managers. WP and VG provided clinical and scientific input. All authors read and approved the final paper.
Corresponding author
Ethics declarations
Conflict of interest
NJ has received research funding from Pharmacyclics, Genentech, AbbVie, ADC Therapeutics, Pfizer, BMS, Verastem, AstraZeneca, Seattle Genetics, Incyte, Celgene, Servier, Cellectis, Adaptive Biotechnologies, Fate Therapeutics, Aprea Therapeutics, and Precision Biosciences. NJ has served on the advisory boards and has received honoraria from Pharmacyclics, Genentech, AbbVie, Adaptive Biotechnologies, TG Therapeutics, Pfizer, Janssen, AstraZeneca, Verastem, Servier, BMS, Beigene, Cellectis, ADC Therapeutics, and Precision Biosciences. VG received research funding from Pharmacyclics, AbbVie, Verastem, Dava Oncology, AstraZeneca, Gilead, Sunesis, and Loxo Oncology. WW has received research support from AbbVie, Pharmacyclics, and Genentech and has received honoraria from Pharmacyclics, AbbVie, Roche, and Genentech. All other authors declare no competing interests.
Ethical approval
The MDACC IRB approved this study and the study was conducted in accordance with the Declaration of Helsinki. This trial was registered on Clinicaltrials.gov (NCT02629809).
Informed consent
All patients provided written informed consent.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Jain, N., Thompson, P., Burger, J. et al. Ibrutinib, fludarabine, cyclophosphamide, and obinutuzumab (iFCG) regimen for chronic lymphocytic leukemia (CLL) with mutated IGHV and without TP53 aberrations. Leukemia 35, 3421–3429 (2021). https://doi.org/10.1038/s41375-021-01280-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41375-021-01280-8
This article is cited by
-
Ibrutinib plus fludarabine, cyclophosphamide and rituximab (iFCR) as initial treatment in chronic lymphocytic leukemia/ small lymphocytic leukemia with or without TP53 aberrations: a prospective real-world study in Chinese cohort
Blood Cancer Journal (2023)
-
Measurable Residual Disease in Chronic Lymphocytic Leukemia: Current Understanding and Evolving Role in Clinical Practice
Current Treatment Options in Oncology (2023)
-
BTK inhibitors in the treatment of hematological malignancies and inflammatory diseases: mechanisms and clinical studies
Journal of Hematology & Oncology (2022)
-
New Treatment Options for Newly-Diagnosed and Relapsed Chronic Lymphocytic Leukemia
Current Treatment Options in Oncology (2022)
-
Measurable residual disease testing in chronic lymphocytic leukaemia: hype, hope neither or both?
Leukemia (2021)