Abstract
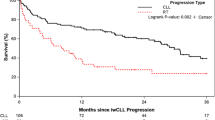
Chronic lymphocytic leukemia (CLL) is associated with perturbed immune function and increased risk for second primary malignancies (SPM). Ibrutinib and acalabrutinib (BTKi) are effective therapies for CLL resulting in partial restoration of immune function. The incidence of and risk factors for SPM in CLL patients receiving BTKi are not yet characterized. We retrospectively determined the incidence of SPM in CLL patients treated with ibrutinib or acalabrutinib at our institution between 2009 and 2017, assessed for association between baseline characteristics and SPM incidence, and compared the observed to expected cancer incidence among age, sex, and year matched controls without CLL. After a median of 44 months follow-up, 64/691 patients (9%) were diagnosed with SPM (excluding non-melanoma skin cancer [NMSC]). The 3-year cumulative incidence rate was 16% for NMSC and 7% for other SPM. On multivariable analysis, smoking was associated with increased SPM risk (HR 2.8 [95% CI: 1.6–4.8]) and higher baseline CD8 count was associated with lower SPM risk (HR 0.9 for 2-fold increase [95% CI: 0.8–0.9]). The observed over expected rate of SPM was 2.2 [95% CI: 1.7–2.9]. CLL patients treated with BTKi remain at increased risk for SPM, and secondary cancer detection is an important consideration in this population.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Miller KD, Siegel RL, Lin CC, Mariotto AB, Kramer JL, Rowland JH, et al. Cancer treatment and survivorship statistics, 2016. CA Cancer J Clin. 2016;66:271–89.
Dasanu CA. Intrinsic and treatment-related immune alterations in chronic lymphocytic leukaemia and their impact for clinical practice. Expert Opin Pharmacother. 2008;9:1481–94.
Perri RT, Kay NE. Abnormal T cell function in early-stage chronic lymphocytic leukemia (CLL) patients. Am J Hematol. 1986;22:55–61.
Schlesinger M, Broman I, Lugassy G. The complement system is defective in chronic lymphatic leukemia patients and in their healthy relatives. Leukemia. 1996;10:1509–13.
Alvarez-Mon M, Casas J, Laguna R, Jorda J, Durantez A. Clinical signification of natural killer activity in B-cell chronic lymphocytic leukemia. Eur J Haematol. 1987;38:268–73.
Forconi F, Moss P. Perturbation of the normal immune system in patients with CLL. Blood. 2015;126:573–81.
Tsimberidou AM, Wen S, McLaughlin P, O’Brien S, Wierda WG, Lerner S, et al. Other malignancies in chronic lymphocytic leukemia/small lymphocytic lymphoma. J Clin Oncol. 2009;27:904–10.
Maurer C, Langerbeins P, Bahlo J, Cramer P, Fink AM, Pflug N, et al. Effect of first-line treatment on second primary malignancies and Richter’s transformation in patients with CLL. Leukemia. 2016;30:2019–25.
Hisada M, Biggar RJ, Greene MH, Fraumeni JF Jr., Travis LB. Solid tumors after chronic lymphocytic leukemia. Blood. 2001;98:1979–81.
Manusow D, Weinerman BH. Subsequent neoplasia in chronic lymphocytic leukemia. JAMA. 1975;232:267–9.
Mellemgaard A, Geisler CH, Storm HH. Risk of kidney cancer and other second solid malignancies in patients with chronic lymphocytic leukemia. Eur J Haematol. 1994;53:218–22.
Royle JA, Baade PD, Joske D, Girschik J, Fritschi L. Second cancer incidence and cancer mortality among chronic lymphocytic leukaemia patients: a population-based study. Br J Cancer. 2011;105:1076–81.
Schollkopf C, Rosendahl D, Rostgaard K, Pipper C, Hjalgrim H. Risk of second cancer after chronic lymphocytic leukemia. Int J Cancer. 2007;121:151–6.
Greene MH, Hoover RN, Fraumeni JF Jr. Subsequent cancer in patients with chronic lymphocytic leukemia–a possible immunologic mechanism. J Natl Cancer Inst. 1978;61:337–40.
Travis LB, Curtis RE, Hankey BF, Fraumeni JF Jr. Second cancers in patients with chronic lymphocytic leukemia. J Natl Cancer Inst. 1992;84:1422–7.
Benjamini O, Jain P, Trinh L, Qiao W, Strom SS, Lerner S, et al. Second cancers in patients with chronic lymphocytic leukemia who received frontline fludarabine, cyclophosphamide and rituximab therapy: distribution and clinical outcomes. Leuk Lymphoma. 2015;56:1643–50.
Lam CC, Ma ES, Kwong YL. Therapy-related acute myeloid leukemia after single-agent treatment with fludarabine for chronic lymphocytic leukemia. Am J Hematol. 2005;79:288–90.
Morrison VA, Rai KR, Peterson BL, Kolitz JE, Elias L, Appelbaum FR, et al. Therapy-related myeloid leukemias are observed in patients with chronic lymphocytic leukemia after treatment with fludarabine and chlorambucil: results of an intergroup study, cancer and leukemia group B 9011. J Clin Oncol. 2002;20:3878–84.
Robak T, Blonski JZ, Gora-Tybor J, Kasznicki M, Konopka L, Ceglarek B, et al. Second malignancies and Richter’s syndrome in patients with chronic lymphocytic leukaemia treated with cladribine. Eur J Cancer. 2004;40:383–9.
Woyach JA, Blachly JS, Rogers KA, Bhat SA, Jianfar M, Lozanski G, et al. Acalabrutinib plus obinutuzumab in treatment-naive and relapsed/refractory chronic lymphocytic leukemia. Cancer Discov. 2020;10:394–405.
Sharman JP, Egyed M, Jurczak W, Skarbnik A, Pagel JM, Flinn IW, et al. Acalabrutinib with or without obinutuzumab versus chlorambucil and obinutuzmab for treatment-naive chronic lymphocytic leukaemia (ELEVATE TN): a randomised, controlled, phase 3 trial. Lancet 2020;395:1278–91.
Bond DA, Woyach JA. Targeting BTK in CLL: beyond Ibrutinib. Curr Hematol Malig Rep. 2019;14:197–205.
O’Brien S, Furman RR, Coutre S, Flinn IW, Burger JA, Blum K, et al. Single-agent ibrutinib in treatment-naive and relapsed/refractory chronic lymphocytic leukemia: a 5-year experience. Blood. 2018;131:1910–9.
Byrd JC, Furman RR, Coutre SE, Burger JA, Blum KA, Coleman M, et al. Three-year follow-up of treatment-naive and previously treated patients with CLL and SLL receiving single-agent ibrutinib. Blood. 2015;125:2497–506.
Byrd JC, Furman RR, Coutre SE, Flinn IW, Burger JA, Blum KA, et al. Targeting BTK with ibrutinib in relapsed chronic lymphocytic leukemia. N Engl J Med. 2013;369:32–42.
Sun C, Tian X, Lee YS, Gunti S, Lipsky A, Herman SE, et al. Partial reconstitution of humoral immunity and fewer infections in patients with chronic lymphocytic leukemia treated with ibrutinib. Blood. 2015;126:2213–9.
Bercusson A, Colley T, Shah A, Warris A, Armstrong-James D. Ibrutinib blocks Btk-dependent NF-kB and NFAT responses in human macrophages during Aspergillus fumigatus phagocytosis. Blood. 2018;132:1985–8.
Fiedler K, Sindrilaru A, Terszowski G, Kokai E, Feyerabend TB, Bullinger L, et al. Neutrophil development and function critically depend on Bruton tyrosine kinase in a mouse model of X-linked agammaglobulinemia. Blood. 2011;117:1329–39.
Lougaris V, Baronio M, Vitali M, Tampella G, Cattalini M, Tassone L, et al. Bruton tyrosine kinase mediates TLR9-dependent human dendritic cell activation. J Allergy Clin Immunol. 2014;133:1644–50 e4.
Ahn IE, Jerussi T, Farooqui M, Tian X, Wiestner A, Gea-Banacloche J. Atypical Pneumocystis jirovecii pneumonia in previously untreated patients with CLL on single-agent ibrutinib. Blood. 2016;128:1940–3.
Ghez D, Calleja A, Protin C, Baron M, Ledoux MP, Damaj G, et al. Early-onset invasive aspergillosis and other fungal infections in patients treated with ibrutinib. Blood. 2018;131:1955–9.
Varughese T, Taur Y, Cohen N, Palomba ML, Seo SK, Hohl TM, et al. Serious infections in patients receiving ibrutinib for treatment of lymphoid cancer. Clin Infect Dis. 2018;67:687–92.
Chamilos G, Lionakis MS, Kontoyiannis DP. Call for action: invasive fungal infections associated with ibrutinib and other small molecule kinase inhibitors targeting immune signaling pathways. Clin Infect Dis. 2018;66:140–8.
Rogers KA, Mousa L, Zhao Q, Bhat SA, Byrd JC, El Boghdadly Z, et al. Incidence of opportunistic infections during ibrutinib treatment for B-cell malignancies. Leukemia. 2019;33:2527–30.
Kyasa MJ, Hazlett L, Parrish RS, Schichman SA, Zent CS. Veterans with chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL) have a markedly increased rate of second malignancy, which is the most common cause of death. Leuk Lymphoma. 2004;45:507–13.
Surveillance, Epidemiology, and End Results (SEER) Program (www.seer.cancer.gov). SEER*Stat Database: incidence- SEER 18 Regs Research Data + Hurricane Katrina Impacted Louisiana Cases, Nov 2018 (Sub 2000–2016) <Katrina/Rita Population Adjustment> -Linked to County Attributes - Total U.S., 1969–2017 Counties, National Cancer Institute, DCCPS, Surveillance Research Program, released April 2019, based on the November 2018 submission.
Sahai H, Khurshid A. Confidence-Intervals for the mean of a poisson-distribution—a review. Biometrical J. 1993;35:857–67.
Surveillance Research Program, National Cancer Institute SEER*Stat software (www.seer.cancer.gov/seerstat) version 8.3.5.
Mulligan SP, Shumack S, Guminski A. Chronic lymphocytic leukemia, skin and other second cancers. Leuk Lymphoma. 2019;60:3104–6.
Mansfield AS, Rabe KG, Slager SL, Schwager SM, Call TG, Brewer JD, et al. Skin cancer surveillance and malignancies of the skin in a community-dwelling cohort of patients with newly diagnosed chronic lymphocytic leukemia. J Oncol Pract. 2014;10:e1–4.
Morton LM, Curtis RE, Linet MS, Bluhm EC, Tucker MA, Caporaso N, et al. Second malignancy risks after non-Hodgkin’s lymphoma and chronic lymphocytic leukemia: differences by lymphoma subtype. J Clin Oncol. 2010;28:4935–44.
Zheng G, Chattopadhyay S, Sud A, Sundquist K, Sundquist J, Forsti A, et al. Second primary cancers in patients with acute lymphoblastic, chronic lymphocytic and hairy cell leukaemia. Br J Haematol. 2019;185:232–9.
Cahoon EK, Pfeiffer RM, Wheeler DC, Arhancet J, Lin SW, Alexander BH, et al. Relationship between ambient ultraviolet radiation and non-Hodgkin lymphoma subtypes: a U.S. population-based study of racial and ethnic groups. Int J Cancer. 2015;136:E432–41.
Morton LM, Hartge P, Holford TR, Holly EA, Chiu BC, Vineis P, et al. Cigarette smoking and risk of non-Hodgkin lymphoma: a pooled analysis from the International Lymphoma Epidemiology Consortium (interlymph). Cancer Epidemiol Biomark Prev. 2005;14:925–33.
Grulich AE, van Leeuwen MT, Falster MO, Vajdic CM. Incidence of cancers in people with HIV/AIDS compared with immunosuppressed transplant recipients: a meta-analysis. Lancet. 2007;370:59–67.
Sigel K, Wisnivesky J, Crothers K, Gordon K, Brown ST, Rimland D, et al. Immunological and infectious risk factors for lung cancer in US veterans with HIV: a longitudinal cohort study. Lancet HIV. 2017;4:e67–e73.
Vajdic CM, McDonald SP, McCredie MR, van Leeuwen MT, Stewart JH, Law M, et al. Cancer incidence before and after kidney transplantation. JAMA. 2006;296:2823–31.
Imai K, Matsuyama S, Miyake S, Suga K, Nakachi K. Natural cytotoxic activity of peripheral-blood lymphocytes and cancer incidence: an 11-year follow-up study of a general population. Lancet. 2000;356:1795–9.
Kesselring A, Gras L, Smit C, van Twillert G, Verbon A, de Wolf F, et al. Immunodeficiency as a risk factor for non-AIDS-defining malignancies in HIV-1-infected patients receiving combination antiretroviral therapy. Clin Infect Dis. 2011;52:1458–65.
Dutta A, Uno H, Lorenz DR, Wolinsky SM, Gabuzda D. Low T-cell subsets prior to development of virus-associated cancer in HIV-seronegative men who have sex with men. Cancer Causes Control. 2018;29:1131–42.
Asgari MM, Ray GT, Quesenberry CP Jr., Katz KA, Silverberg MJ. Association of multiple primary skin cancers with human immunodeficiency virus infection, CD4 count, and viral load. JAMA Dermatol. 2017;153:892–6.
Younes A, Brody J, Carpio C, Lopez-Guillermo A, Ben-Yehuda D, Ferhanoglu B, et al. Safety and activity of ibrutinib in combination with nivolumab in patients with relapsed non-Hodgkin lymphoma or chronic lymphocytic leukaemia: a phase 1/2a study. Lancet Haematol. 2019;6:e67–e78.
Jain N, Basu S, Thompson P, Ohanian M, Ferrajoli A, Pemmaraju N, et al. Nivolumab combined with Ibrutinib for CLL and richter transformation: a Phase II Trial. Blood. 2016;128:59.
Witzig TE, Maddocks K, De Vos S, Lyons RM, Edenfield J, Sharman J, et al. Phase 1/2 trial of acalabrutinib plus pembrolizumab in relapsed/ refractory diffuse large B-cell lymphoma. J Clin Oncol. 2019;37:7519.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
KAR has consulted for Acerta Pharma, AstraZeneca, and Pharmacyclics, has received travel funding from Astra Zeneca, and has received research funding from Genentech, AbbVie, and Janssen. SAB has consulted for Pharmacyclics and Janssen. MRG has served on an advisory committee for Acerta Pharma. SMJ has received research funding from Pharmacyclics. JCB has consulted for Janssen, received travel support from TG Therapeutics, Janssen, Pharmacyclics, and Gilead, served on speakers bureau for Gilead, Pharmacyclics, Janssen, Novartis, and TG Therapeutics, and received research funding from Gilead, Acerta, BeiGene, Janssen, Genentech, Pharmacyclics, and TG Therapeutics. JAW has consulted for Janssen, Pharmacyclics, AstraZeneca, and Arqule and has received research funding from Janssen, Pharmacyclics, Loxo, and Arqule. DAB, YH, JLF, ASR, DHO, EMB, and KJM have no relevant competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Bond, D.A., Huang, Y., Fisher, J.L. et al. Second cancer incidence in CLL patients receiving BTK inhibitors. Leukemia 34, 3197–3205 (2020). https://doi.org/10.1038/s41375-020-0987-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41375-020-0987-6
This article is cited by
-
Risk of second primary malignancies in patients with chronic lymphocytic leukemia: a population-based study in the Netherlands, 1989-2019
Blood Cancer Journal (2023)
-
Managing Waldenström’s macroglobulinemia with BTK inhibitors
Leukemia (2023)
-
Clinical features and prognosis of double primary malignant neoplasms in patients with non-hodgkin lymphoma
Discover Oncology (2023)
-
Second primary malignancies in non-Hodgkin lymphoma: epidemiology and risk factors
Annals of Hematology (2023)
-
Restoration of the immune function as a complementary strategy to treat Chronic Lymphocytic Leukemia effectively
Journal of Experimental & Clinical Cancer Research (2021)