Abstract
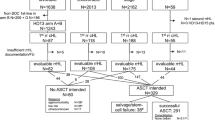
Richter transformation (RT) is defined as development of aggressive lymphoma in patients (pts) with CLL. The incidence rates of RT among pts with CLL range from 2 to 10%. The aim of this analysis is to report the frequency, characteristics and outcomes of pts with RT enrolled in trials of the GCLLSG. A total of 2975 pts with advanced CLL were reviewed for incidence of RT. Clinical, laboratory, and genetic data were pooled. Time-to-event data, starting from time of CLL diagnosis, of first-line therapy or of RT diagnosis, were analyzed by Kaplan-Meier methodology. One hundred and three pts developed RT (3%): 95 pts diffuse large B-cell lymphoma (92%) and eight pts Hodgkin lymphoma (8%). Median observation time was 53 months (interquartile range 38.1–69.5). Median OS from initial CLL diagnosis for pts without RT was 167 months vs 71 months for pts with RT (HR 2.64, CI 2.09–3.33). Median OS after diagnosis of RT was 9 months. Forty-seven pts (46%) received CHOP-like regimens for RT treatment. Three pts subsequently underwent allogeneic and two pts autologous stem cell transplantation. Our findings show that within a large cohort of GCLLSG trial participants, 3% of the pts developed RT after receiving first-line chemo- or chemoimmunotherapy. This dataset confirms the ongoing poor prognosis and high mortality associated with RT.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Rossi D, Spina V, Gaidano G. Biology and treatment of Richter syndrome. Blood. 2018;131:2761–72.
Rossi D, Spina V, Deambrogi C, Rasi S, Laurenti L, Stamatopoulos K, et al. The genetics of Richter syndrome reveals disease heterogeneity and predicts survival after transformation. Blood. 2011;117:3391–401.
Swerdlow SH. WHO classification of tumours of haematopoietic and lymphoid tissues. WHO classification tumours 2008. 2008;22008:439.
Bockorny B, Codreanu I, Dasanu CA. Hodgkin lymphoma as Richter transformation in chronic lymphocytic leukaemia: a retrospective analysis of world literature. Br J Haematol. 2012;156:50–66.
Brecher M, Banks PM. Hodgkin’s disease variant of Richter’s syndrome. Report of eight cases. Am J Clin Pathol. 1990;93:333–9.
Mato AR, Wierda WG, Davids MS, Cheson BD, Coutre SE, Choi M, et al. Utility of PET-CT in patients with chronic lymphocytic leukemia following B-cell receptor pathway inhibitor therapy. Haematologica. 2019;104:2258–64.
Federmann B, Mueller MR, Steinhilber J, Horger MS, Fend F. Diagnosis of Richter transformation in chronic lymphocytic leukemia: histology tips the scales. Ann Hematol. 2018;97:1859–68.
Mao Z, Quintanilla-Martinez L, Raffeld M, Richter M, Krugmann J, Burek C, et al. IgVH mutational status and clonality analysis of Richter’s transformation: diffuse large B-cell lymphoma and Hodgkin lymphoma in association with B-cell chronic lymphocytic leukemia (B-CLL) represent 2 different pathways of disease evolution. Am J Surg Pathol. 2007;31:1605–14.
Behdad A, Griffin B, Chen YH, Ma S, Kelemen K, Lu X, et al. PD-1 is highly expressed by neoplastic B-cells in Richter transformation. Br J Haematol. 2018;185:370–3.
He R, Ding W, Viswanatha DS, Chen D, Shi M, Van Dyke D, et al. PD-1 expression in chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL) and large B-cell richter transformation (DLBCL-RT): a characteristic feature of DLBCL-RT and potential surrogate marker for clonal relatedness. Am J Surg Pathol. 2018;42:843–54.
Parikh SA, Rabe KG, Call TG, Zent CS, Habermann TM, Ding W, et al. Diffuse large B-cell lymphoma (Richter syndrome) in patients with chronic lymphocytic leukaemia (CLL): a cohort study of newly diagnosed patients. Br J Haematol. 2013;162:774–82.
Tsimberidou AM, O’Brien S, Khouri I, Giles FJ, Kantarjian HM, Champlin R, et al. Clinical outcomes and prognostic factors in patients with Richter’s syndrome treated with chemotherapy or chemoimmunotherapy with or without stem-cell transplantation. J Clin Oncol Off J Am Soc Clin Oncol. 2006;24(May):2343–51.
Harousseau JL, Flandrin G, Tricot G, Brouet JC, Seligmann M, Bernard J. Malignant lymphoma supervening in chronic lymphocytic leukemia and related disorders. Richter’s syndrome: a study of 25 cases. Cancer. 1981;48:1302–8.
Robertson LE, Pugh W, O’Brien S, Kantarjian H, Hirsch-Ginsberg C, Cork A, et al. Richter’s syndrome: a report on 39 patients. J Clin Oncol Off J Am Soc Clin Oncol. 1993;11:1985–9.
Tsimberidou AM, Keating MJ, Wierda WG. Richter’s transformation in chronic lymphocytic leukemia. Curr Hematol Malig Rep. 2007;2(Oct):265–71.
Jain N, Keating MJ. Richter transformation of CLL. Expert Rev Hematol. 2016;9:793–801.
Parikh SA, Kay NE, Shanafelt TD. How we treat Richter syndrome. Blood. 2014;123:1647–57.
Ding W. Richter transformation in the era of novel agents. Hematol Am Soc Hematol Educ Program. 2018;2018:256–63.
Wang Y, Tschautscher MA, Rabe KG, Call TG, Leis JF, Kenderian SS, et al. Clinical characteristics and outcomes of Richter transformation: Experience of 204 patients from a single center. Haematologica 2019;105:765–73.
Cramer P, von Tresckow J, Bahlo J, Robrecht S, Langerbeins P, Al-Sawaf O, et al. Bendamustine followed by obinutuzumab and venetoclax in chronic lymphocytic leukaemia (CLL2-BAG): primary endpoint analysis of a multicentre, open-label, phase 2 trial. Lancet Oncol. 2018;19:1215–28.
Eichhorst B, Fink AM, Bahlo J, Busch R, Kovacs G, Maurer C, et al. First-line chemoimmunotherapy with bendamustine and rituximab versus fludarabine, cyclophosphamide, and rituximab in patients with advanced chronic lymphocytic leukaemia (CLL10): an international, open-label, randomised, phase 3, non-inferiority trial. Lancet Oncol. 2016;17:928–42.
Eichhorst BF, Busch R, Hopfinger G, Pasold R, Hensel M, Steinbrecher C, et al. Fludarabine plus cyclophosphamide versus fludarabine alone in first-line therapy of younger patients with chronic lymphocytic leukemia. Blood. 2006;107:885–91.
Eichhorst BF, Busch R, Stilgenbauer S, Stauch M, Bergmann MA, Ritgen M, et al. First-line therapy with fludarabine compared with chlorambucil does not result in a major benefit for elderly patients with advanced chronic lymphocytic leukemia. Blood. 2009;114:3382–91.
Fischer K, Cramer P, Busch R, Bottcher S, Bahlo J, Schubert J, et al. Bendamustine in combination with rituximab for previously untreated patients with chronic lymphocytic leukemia: a multicenter phase II trial of the German Chronic Lymphocytic Leukemia Study Group. J Clin Oncol Off J Am Soc Clin Oncol. 2012;30:3209–16.
Goede V, Fischer K, Busch R, Engelke A, Eichhorst B, Wendtner CM, et al. Obinutuzumab plus chlorambucil in patients with CLL and coexisting conditions. N Engl J Med. 2014;370:1101–10.
Hallek M, Fischer K, Fingerle-Rowson G, Fink AM, Busch R, Mayer J, et al. Addition of rituximab to fludarabine and cyclophosphamide in patients with chronic lymphocytic leukaemia: a randomised, open-label, phase 3 trial. Lancet. 2010;376:1164–74.
von Tresckow J, Cramer P, Bahlo J, Robrecht S, Langerbeins P, Fink AM, et al. CLL2-BIG: sequential treatment with bendamustine, ibrutinib and obinutuzumab (GA101) in chronic lymphocytic leukemia. Leukemia. 2019;33:1161–72.
Stilgenbauer S, Schnaiter A, Paschka P, Zenz T, Rossi M, Dohner K, et al. Gene mutations and treatment outcome in chronic lymphocytic leukemia: results from the CLL8 trial. Blood. 2014;123:3247–54.
Rossi D, Spina V, Cerri M, Rasi S, Deambrogi C, De Paoli L, et al. Stereotyped B-cell receptor is an independent risk factor of chronic lymphocytic leukemia transformation to Richter syndrome. Clin Cancer Res Off J Am Assoc Cancer Res. 2009;15:4415–22.
Rossi D, Cerri M, Capello D, Deambrogi C, Rossi FM, Zucchetto A, et al. Biological and clinical risk factors of chronic lymphocytic leukaemia transformation to Richter syndrome. Br J Haematol. 2008;142:202–15.
Yee KW, O’Brien SM, Giles FJ. Richter’s syndrome: biology and therapy. Cancer J. 2005;11:161–74.
Cheson BD, Vena DA, Barrett J, Freidlin B. Second malignancies as a consequence of nucleoside analog therapy for chronic lymphoid leukemias. J Clin Oncol Off J Am Soc Clin Oncol. 1999;17:2454–60.
Robak T, Blonski JZ, Gora-Tybor J, Kasznicki M, Konopka L, Ceglarek B, et al. Second malignancies and Richter’s syndrome in patients with chronic lymphocytic leukaemia treated with cladribine. Eur J Cancer. 2004;40:383–9.
Mauro FR, Foa R, Giannarelli D, Cordone I, Crescenzi S, Pescarmona E, et al. Clinical characteristics and outcome of young chronic lymphocytic leukemia patients: a single institution study of 204 cases. Blood. 1999;94:448–54.
Stilgenbauer S, Zenz T, Winkler D, Buhler A, Schlenk RF, Groner S, et al. Subcutaneous alemtuzumab in fludarabine-refractory chronic lymphocytic leukemia: clinical results and prognostic marker analyses from the CLL2H study of the German Chronic Lymphocytic Leukemia Study Group. J Clin Oncol Off J Am Soc Clin Oncol. 2009;27:3994–4001.
Tam CS, Shanafelt TD, Wierda WG, Abruzzo LV, Van Dyke DL, O’Brien S, et al. De novo deletion 17p13.1 chronic lymphocytic leukemia shows significant clinical heterogeneity: the M. D. Anderson and Mayo Clinic experience. Blood. 2009;114:957–64.
Rossi D, Gaidano G. Biological and clinical significance of stereotyped B-cell receptors in chronic lymphocytic leukemia. Haematologica. 2010;95:1992–5.
Dabaja BS, O’Brien SM, Kantarjian HM, Cortes JE, Thomas DA, Albitar M, et al. Fractionated cyclophosphamide, vincristine, liposomal daunorubicin (daunoXome), and dexamethasone (hyperCVXD) regimen in Richter’s syndrome. Leuk lymphoma. 2001;42:329–37.
Tsimberidou AM, Wierda WG, Wen S, Plunkett W, O’Brien S, Kipps TJ, et al. Phase I-II clinical trial of oxaliplatin, fludarabine, cytarabine, and rituximab therapy in aggressive relapsed/refractory chronic lymphocytic leukemia or Richter syndrome. Clin Lymphoma Myeloma Leuk. 2013;13:568–74.
Langerbeins P, Busch R, Anheier N, Durig J, Bergmann M, Goebeler ME, et al. Poor efficacy and tolerability of R-CHOP in relapsed/refractory chronic lymphocytic leukemia and Richter transformation. Am J Hematol. 2014;89:E239–43.
Abrisqueta P, Delgado J, Alcoceba M, González M, Oliveira AC, Loscertales J, et al. Clinical Outcome and Prognostic Factors of Patients with Richter’s Syndrome: Retrospective Multicenter Study of the Spanish Chronic Lymphocytic Leukemia (CLL) Study Group (GELLC). Blood. 2017;130(Suppl 1):2995.
Burger JA, Barr PM, Robak T, Owen C, Ghia P, Tedeschi A, et al. Long-term efficacy and safety of first-line ibrutinib treatment for patients with CLL/SLL: 5 years of follow-up from the phase 3 RESONATE-2 study. Leukemia. 2019;34:787–98.
Fischer K, Al-Sawaf O, Bahlo J, Fink AM, Tandon M, Dixon M, et al. Venetoclax and obinutuzumab in patients with CLL and coexisting conditions. N Engl J Med. 2019;380:2225–36.
Woyach JA, Ruppert AS, Heerema NA, Zhao W, Booth AM, Ding W, et al. Ibrutinib regimens versus chemoimmunotherapy in older patients with untreated CLL. N Engl J Med. 2018;379:2517–28.
Ding W, Le-Rademacher J, Call TG, Parikh SA, Leis JF, Shanafelt TD, et al. PD-1 blockade with pembrolizumab in relapsed CLL including Richter’s transformation: an updated report from a phase 2 trial (MC1485). Blood. 2016;128:4392.
Gauthier J, Hirayama AV, Hay KA, Li D, Lymp J, Sheih A, et al. Comparison of efficacy and toxicity of CD19-specific chimeric antigen receptor T-cells alone or in combination with ibrutinib for relapsed and/or refractory CLL. Blood. 2018;132(Suppl 1):299.
Gill SI, Vides V, Frey NV, Metzger S, O’Brien M, Hexner E, et al. Prospective clinical trial of anti-CD19 CAR T cells in combination with ibrutinib for the treatment of chronic lymphocytic leukemia shows a high response rate. Blood. 2018;132(Suppl 1):298.
Jain N, Ferrajoli A, Basu S, Thompson PA, Burger JA, Kadia TM, et al. A phase II trial of nivolumab combined with ibrutinib for patients with richter transformation. Blood. 2018;132(Suppl 1):296.
Acknowledgements
We thank the patients and their families for the trust and confidence they have placed in us as well as all participating sites for their active contributions to our studies. OA, SR, JB, BE designed the study, analysed the data and wrote the manuscript. AMF, PC, JvT, EL, MK, MD, MR, JD, ET, SS, CMW, VG and MH revised and discussed the data and reviewed the manuscript. ET and StSt were supported by the DFG (SFB1074, subprojects B1 and B2).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
OA reports personal fees and non-financial support from AbbVie, Roche, Gilead, Janssen. JB reports personal fees and non-financial support from Roche. PC reports personal fees and non-financial support from Roche, Janssen, Astellas, Gilead, Mundipharma, Novartis. JD reports personal fees and non-financial support from Celgene, Janssen and Abbvie. BE reports grants and personal fees from Roche, AbbVie, Gilead, Janssen. AMF reports personal fees from Janssen Pharmaceutical. KF reports non-financial support and personal fees from Roche and Abbvie. VG reports personal fees and non-financial support from Roche, Gilead, Janssen. MH reports personal fees and non-financial support from AbbVie, Roche, Gilead, Janssen, personal fees from Celgene, Boehringer Ingelheim. MR reports grants and personal fees from Roche/AbbVie. StSt reports grants, personal fees and non-financial support from AbbVie, Roche; grants, personal fees and non-financial support from Amgen, AstraZeneca, Celgene, Gilead, GSK, Janssen, Novartis, Pharmacyclics, Sunesis. ET reports personal fees from Roche, AbbVie. CMW reports grants and/or personal fees from Hoffmann-La Roche, Mundipharma, Servier; Janssen-Cilag, Novartis, Gilead, Morphosys and Abbvie. All others declare no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Al-Sawaf, O., Robrecht, S., Bahlo, J. et al. Richter transformation in chronic lymphocytic leukemia (CLL)—a pooled analysis of German CLL Study Group (GCLLSG) front line treatment trials. Leukemia 35, 169–176 (2021). https://doi.org/10.1038/s41375-020-0797-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41375-020-0797-x
This article is cited by
-
Venetoclax plus dose-adjusted R-EPOCH (VR-DA-EPOCH) or G-EPOCH bridging to subsequent cellular therapy for the patients with transformed lymphoma a single center clinical experience
Annals of Hematology (2024)
-
Treatment of Richter’s Transformation with Novel Therapies
Current Hematologic Malignancy Reports (2024)
-
Tislelizumab plus zanubrutinib for Richter transformation: the phase 2 RT1 trial
Nature Medicine (2024)
-
Allogeneic hematopoietic cell transplantation for Richter transformation of chronic lymphocytic leukemia: an intention-to-transplant analysis
Bone Marrow Transplantation (2023)
-
Immunochemotherapy combined with novel agents for Richter syndrome: report of 3 cases
Holistic Integrative Oncology (2023)