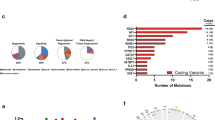
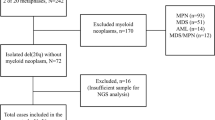
Abstract
Therapy-related myeloid neoplasms (tMN) following successful treatment of acute myeloid leukemia (AML) are rare and poorly characterized. To evaluate the presence of a common ancestral clone, we performed whole-exome sequencing of 25 patients at AML diagnosis, tMN diagnosis (tMDS: 13; tAML: 12), and matched remission samples, identifying 607 mutations affecting 504 different genes (46 recurrently mutated). Number of mutations was higher in tAML vs. tMDS cases (median 19 vs 13 mutations, p = 0.05). Focusing on 24 genes commonly mutated in hematological malignancies, 19/25 (76%) patients were found to share mutations between AML and tMN, mostly affecting epigenetic modifiers (21/32; 66%), splicing factors (6/32; 19%), and chromatin modifiers (3/32; 9%). Analysis of remission samples identified 13 persisting mutations in 10/22 patients, affecting DNMT3A (n = 6), TET2 (n = 5), IDH1 and SRSF2 (n = 1, each). Comparison of cytogenetics revealed that 9/12 patients with a normal karyotype (NK) in AML harbored aberrations in tMN, four aberrant AML cases presented with NK in tMN, four other patients showed unrelated cytogenetic aberrations. Our study provides novel insights into the pathogenesis of tMN, hypothesizing the presence of a common ancestral clone in AML and tMN. Mutations mostly affected epigenetic modifiers, which have previously been linked to clonal hematopoiesis.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Arber DA, Orazi A, Hasserjian R, Thiele J, Borowitz MJ, Le Beau MM, et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016;127:2391–405.
McNerney ME, Godley LA, Le Beau MM. Therapy-related myeloid neoplasms: when genetics and environment collide. Nat Rev Cancer. 2017;17:513–27.
Fianchi L, Pagano L, Piciocchi A, Candoni A, Gaidano G, Breccia M, et al. Characteristics and outcome of therapy-related myeloid neoplasms: report from the Italian network on secondary leukemias. Am J Hematol. 2015;90:E80–85.
Larson RA. Therapy-related myeloid neoplasms. Haematologica. 2009;94:454–9.
Kayser S, Döhner K, Krauter J, Köhne CH, Horst HA, Held G.German-Austrian AMLSG et al. The impact of therapy-related acute myeloid leukemia (AML) on outcome in 2853 adult patients with newly diagnosed AML. Blood. 2011;117:2137–45.
Lindsley RC, Mar BG, Mazzola E, Graumann PV, Shareef S, Allen SL, et al. Acute myeloid leukemia ontogeny is defined by distinct somatic mutations. Blood. 2015;125:1367–76.
Ley TJ, Miller C, Ding L, Raphael BL, Mungall AJ, Robertson A. Cancer Genome Atlas Research Network et al. Genomic and epigenomic landscapes of adult de novo acute myeloid leukemia. N Engl J Med. 2013;368:2059–74.
Metzeler KH, Herold T, Rothenberg-Thurley M, Amler S, Sauerland MC, Görlich D, et al. Spectrum and prognostic relevance of driver gene mutations in acute myeloid leukemia. Blood. 2016;128:686–98. AMLCG Study Group.
Churpek JE, Marquez R, Neistadt B, Claussen K, Lee MK, Churpek MM, et al. Inherited mutations in cancer susceptibility genes are common among survivors of breast cancer who develop therapy-related leukemia. Cancer. 2016;122:304–11.
Schulz E, Valentin A, Ulz P, Beham-Schmid C, Lind K, Rupp V, et al. Germline mutations in the DNA damage response genes BRCA1, BRCA2, BARD1 and TP53 in patients with therapy related myeloid neoplasms. J Med Genet. 2012;49:422–8.
Voso MT, Fabiani E, Zang Z, Fianchi L, Falconi G, Padella A, et al. Fanconi anemia gene variants in therapy-related myeloid neoplasms. Blood Cancer J. 2015;3:5:e323.
Takahashi K, Wang F, Kantarjian H, Doss D, Khanna K, Thompson E, et al. Preleukaemic clonal haemopoiesis and risk of therapy-related myeloid neoplasms: a case-control study. Lancet Oncol. 2017;18:100–11.
Gillis NK, Ball M, Zhang Q, Ma Z, Zhao Y, Yoder SJ, et al. Clonal haemopoiesis and therapy-related myeloid malignancies in elderly patients: a proof-of-concept, case-control study. Lancet Oncol. 2017;18:112–21.
Gaut D, Sasine J, Schiller G. Secondary clonal hematologic neoplasia following successful therapy for acute promyelocytic leukemia (APL): A report of two cases and review of the literature. Leuk Res Rep. 2018;9:65–71.
Göhring G, Thomay K, Schmidt G, Ripperger T, Xu M, Wittner N, et al. A common ancestral DNMT3A-mutated preleukemic clone giving rise to AML and MDS in an adolescent girl. Leuk Lymphoma. 2017;58:718–21.
Kim HG, Jang JH, Koh EH. TRIP11-PDGFRB fusion in a patient with a therapy-related myeloid neoplasm with t(5;14)(q33;q32) after treatment for acute promyelocytic leukemia. Mol Cytogenet. 2014;7:103.
Herold S, Sockel K, Sayehli C, Herbst R, Dührsen U, Oelschlägel U, et al. Evolution of NPM1-negative therapy-related myelodysplastic syndromes following curative treatment of NPM1-mutant AML. Leukemia. 2017;31:2247–51.
Schoch C, Schnittger S, Bursch S, Gerstner D, Hochhaus A, Berger U, et al. Comparison of chromosome banding analysis, interphase- and hypermetaphase-FISH, qualitative and quantitative PCR for diagnosis and for follow-up in chronic myeloid leukemia: a study on 350 cases. Leukemia. 2002;16:53–9.
Haferlach T, Kern W, Schoch C, Hiddemann W, Sauerland MC. Morphologic dysplasia in acute myeloid leukemia: importance of granulocytic dysplasia. J Clin Oncol. 2003;21:3004–5.
Kern W, Voskova D, Schoch C, Hiddemann W, Schnittger S, Haferlach T. Determination of relapse risk based on assessment of minimal residual disease during complete remission by multiparameter flow cytometry in unselected patients with acute myeloid leukemia. Blood. 2004;104:3078–85.
Höllein A, Meggendorfer M, Dicker F, Jeromin S, Nadarajah N, Kern W, et al. NPM1 mutated AML can relapse with wild-type NPM1: persistent clonal hematopoiesis can drive relapse. Blood Adv. 2018;2:3118–25.
Fuentes Fajardo KV, Adams D, Mason CE, Sincan M, Tifft C, Toro C. NISC Comparative Sequencing Program et al. Detecting false-positive signals in exome sequencing. Hum Mutat. 2012;33:609–13.
Adzhubei IA, Schmidt S, Peshkin L, Ramensky VE, Gerasimova A, Bork P, et al. A method and server for predicting damaging missense mutations. Nat Methods. 2010;7:248–9.
Döhner H, Weisdorf DJ, Bloomfield CD. Acute myeloid leukemia. N Engl J Med. 2015;373:1136–52.
Knijnenburg TA, Wang L, Zimmermann MT, Chambwe N, Gao GF, Cherniack AD, et al. Genomic and molecular landscape of DNA damage repair deficiency across the cancer genome atlas. Cell Rep. 2018;23:239–54.
Dorsman JC, Levitus M, Rockx D, Rooimans MA, Oostra AB, Haitjema A, et al. Identification of the Fanconi anemia complementation group I gene, FANCI. Cell Oncol. 2007;29:211–8.
Cocciardi S, Dolnik A, Kapp-Schwoerer S, Rücker FG, Lux S, Blätte TJ, et al. Clonal evolution patterns in acute myeloid leukemia with NPM1 mutation. Nat Commun. 2019;10:2031.
Majeti R. Clonal evolution of pre-leukemic hematopoietic stem cells precedes human acute myeloid leukemia. Best Pr Res Clin Haematol. 2014;27:229–34.
Chan SM, Majeti R. Role of DNMT3A, TET2, and IDH1/2 mutations in pre-leukemic stem cells in acute myeloid leukemia. Int J Hematol. 2013;98:648–57.
Corces-Zimmerman MR, Hong WJ, Weissman IL, Medeiros BC, Majeti R. Preleukemic mutations in human acute myeloid leukemia affect epigenetic regulators and persist in remission. Proc Natl Acad Sci USA. 2014;111:2548–53.
Renneville A, Attias P, Thomas X, Bally C, Hayette S, Farhat H, et al. Genetic analysis of therapy-related myeloid neoplasms occurring after intensive treatment for acute promyelocytic leukemia. Leukemia. 2018;32:2066–9.
Le Beau MM, Davis EM, Patel B, Phan VT, Sohal J, Kogan SC. Recurring chromosomal abnormalities in leukemia in PML-RARA transgenic mice identify cooperating events and genetic pathways to acute promyelocytic leukemia. Blood. 2003;102:1072–4.
Wartman LD, Larson DE, Xiang Z, Ding L, Chen K, Lin L, et al. Sequencing a mouse acute promyelocytic leukemia genome reveals genetic events relevant for disease progression. J Clin Invest. 2011;121:1445–55.
Castilla LH, Perrat P, Martinez NJ, Landrette SF, Keys R, Oikemus S, et al. Identification of genes that synergize with Cbfb-MYH11 in the pathogenesis of acute myeloid leukemia. Proc Natl Acad Sci USA. 2004;101:4924–9.
Duployez N, Marceau-Renaut A, Boissel N, Petit A, Bucci M, Geffroy S, et al. Comprehensive mutational profiling of core binding factor acute myeloid leukemia. Blood. 2016;127:2451–9.
Rothenberg-Thurley M, Amler S, Goerlich D, Köhnke T, Konstandin NP, Schneider S et al. Persistence of pre-leukemic clones during first remission and risk of relapse in acute myeloid leukemia. Leukemia. 2017; https://doi.org/10.1038/leu.2017.350.
Morita K, Kantarjian HM, Wang F, Yan Y, Bueso-Ramos C, Sasaki K, et al. Clearance of somatic mutations at remission and the risk of relapse in acute myeloid leukemia. J Clin Oncol. 2018;36:1788–97.
Klco JM, Miller CA, Griffith M, Petti A, Spencer DH, Ketkar-Kulkarni S, et al. Association between mutation clearance after induction therapy and outcomes in acute myeloid leukemia. JAMA. 2015;314:811–22.
Acknowledgements
We thank all co-workers at the MLL Munich Leukemia Laboratory for approaching together many aspects in the field of leukemia diagnostics and research by their dedicated work. The authors would like to thank all physicians for providing samples and caring for patients as well as collecting data.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
CH, WK and TH declare part ownership of MLL Munich Leukemia Laboratory. LH, NN, MM, AH, CV and AS are employed by the MLL Munich Leukemia Laboratory. The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Hartmann, L., Nadarajah, N., Meggendorfer, M. et al. Molecular characterization of a second myeloid neoplasm developing after treatment for acute myeloid leukemia. Leukemia 34, 811–820 (2020). https://doi.org/10.1038/s41375-019-0633-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41375-019-0633-3