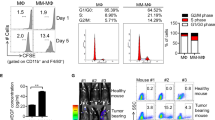
Abstract
Multiple myeloma is the second most frequent hematological cancer after lymphoma and remains an incurable disease. The pervasive support provided by the bone marrow microenvironment to myeloma cells is crucial for their survival. Here, an unbiased assessment of receptor tyrosine kinases overexpressed in myeloma identified ROR2, a receptor for the WNT noncanonical pathway, as highly expressed in myeloma cells. Its ligand, WNT5A is the most abundant growth factor in the bone marrow of myeloma patients. ROR2 mediates myeloma cells interactions with the surrounding bone marrow and its depletion resulted in detachment of myeloma cells from their niche in an in vivo model, triggering apoptosis and thus markedly delaying disease progression. Using in vitro and ex vivo 3D-culture systems, ROR2 was shown to exert a pivotal role in the adhesion of cancer cells to the microenvironment. Genomic studies revealed that the pathways mostly deregulated by ROR2 overexpression were PI3K/AKT and mTOR. Treatment of cells with specific PI3K inhibitors already used in the clinic reduced myeloma cell adhesion to the bone marrow. Together, our findings support the view that ROR2 and its downstream targets represent a novel therapeutic strategy for the large subgroup of MM patients whose cancer cells show ROR2 overexpression.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Smith D, Yong K. Advances in understanding prognosis in myeloma. Br J Haematol. 2016;175:367–80.
Mondello P, Cuzzocrea S, Navarra M, Mian M. Bone marrow micro-environment is a crucial player for myelomagenesis and disease progression. Oncotarget. 2017;8:20394–409.
Hideshima T, Mitsiades C, Tonon G, Richardson PG, Anderson KC. Understanding multiple myeloma pathogenesis in the bone marrow to identify new therapeutic targets. Nat Rev Cancer. 2007;7:585–98.
Jansen M, de Leng WW, Baas AF, Myoshi H, Mathus-Vliegen L, Taketo MM, et al. Mucosal prolapse in the pathogenesis of Peutz-Jeghers polyposis. Gut. 2006;55:1–5.
Azab AK, Runnels JM, Pitsillides C, Moreau AS, Azab F, Leleu X, et al. CXCR4 inhibitor AMD3100 disrupts the interaction of multiple myeloma cells with the bone marrow microenvironment and enhances their sensitivity to therapy. Blood. 2009;113:4341–51.
Loh KM, van Amerongen R, Nusse R. Generating cellular diversity and spatial form: Wnt signaling and the evolution of multicellular animals. Dev Cell. 2016;38:643–55.
Mahtouk K, Moreaux J, Hose D, Reme T, Meissner T, Jourdan M, et al. Growth factors in multiple myeloma: a comprehensive analysis of their expression in tumor cells and bone marrow environment using Affymetrix microarrays. BMC Cancer. 2010;10:198.
Semenov MV, Habas R, Macdonald BT, He X. SnapShot: noncanonical Wnt signaling pathways. Cell. 2007;131:1378.
van Amerongen R. Alternative Wnt pathways and receptors. Cold Spring Harb Perspect Biol. 2012;4:a007914.
Zallen JA. Planar polarity and tissue morphogenesis. Cell. 2007;129:1051–63.
Sedgwick AE, D’Souza-Schorey C. Wnt signaling in cell motility and invasion: drawing parallels between development and cancer. Cancers (Basel). 2016;8,80.
VanderVorst K, Hatakeyama J, Berg A, Lee H, Carraway KL 3rd. Cellular and molecular mechanisms underlying planar cell polarity pathway contributions to cancer malignancy. Semin Cell Dev Biol. 2018;81:78–87.
Shojima K, Sato A, Hanaki H, Tsujimoto I, Nakamura M, Hattori K, et al. Wnt5a promotes cancer cell invasion and proliferation by receptor-mediated endocytosis-dependent and -independent mechanisms, respectively. Sci Rep. 2015;5:8042.
Bo H, Gao L, Chen Y, Zhang J, Zhu M. Upregulation of the expression of Wnt5a promotes the proliferation of pancreatic cancer cells in vitro and in a nude mouse model. Mol Med Rep. 2016;13:1163–71.
Yu J, Chen L, Cui B, Widhopf GF 2nd, Shen Z, Wu R, et al. Wnt5a induces ROR1/ROR2 heterooligomerization to enhance leukemia chemotaxis and proliferation. J Clin Investig. 2016;126:585–98.
Yamamoto H, Yoo SK, Nishita M, Kikuchi A, Minami Y. Wnt5a modulates glycogen synthase kinase 3 to induce phosphorylation of receptor tyrosine kinase Ror2. Genes Cells. 2007;12:1215–23.
Akbarzadeh S, Wheldon LM, Sweet SM, Talma S, Mardakheh FK, Heath JK. The deleted in brachydactyly B domain of ROR2 is required for receptor activation by recruitment of Src. PloS One. 2008;3:e1873.
Takeuchi S, Takeda K, Oishi I, Nomi M, Ikeya M, Itoh K, et al. Mouse Ror2 receptor tyrosine kinase is required for the heart development and limb formation. Genes Cells. 2000;5:71–8.
Debebe Z, Rathmell WK. Ror2 as a therapeutic target in cancer. Pharmacol Ther. 2015;150:143–8.
Lara E, Calvanese V, Huidobro C, Fernandez AF, Moncada-Pazos A, Obaya AJ, et al. Epigenetic repression of ROR2 has a Wnt-mediated, pro-tumourigenic role in colon cancer. Mol Cancer. 2010;9:170.
Ma SS, Srivastava S, Llamosas E, Hawkins NJ, Hesson LB, Ward RL, et al. ROR2 is epigenetically inactivated in the early stages of colorectal neoplasia and is associated with proliferation and migration. BMC Cancer. 2016;16:508.
Belloni D, Heltai S, Ponzoni M, Villa A, Vergani B, Pecciarini L, et al. Modeling multiple myeloma-bone marrow interactions and response to drugs in a 3D surrogate microenvironment. Haematologica. 2018;103:707–16.
Zhan F, Huang Y, Colla S, Stewart JP, Hanamura I, Gupta S, et al. The molecular classification of multiple myeloma. Blood. 2006;108:2020–8.
Claudio JO, Zhan F, Zhuang L, Khaja R, Zhu YX, Sivananthan K, et al. Expression and mutation status of candidate kinases in multiple myeloma. Leukemia. 2007;21:1124–7.
Rozemuller H, van der Spek E, Bogers-Boer LH, Zwart MC, Verweij V, Emmelot M, et al. A bioluminescence imaging based in vivo model for preclinical testing of novel cellular immunotherapy strategies to improve the graft-versus-myeloma effect. Haematologica. 2008;93:1049–57.
Santo L, Hideshima T, Kung AL, Tseng JC, Tamang D, Yang M, et al. Preclinical activity, pharmacodynamic, and pharmacokinetic properties of a selective HDAC6 inhibitor, ACY-1215, in combination with bortezomib in multiple myeloma. Blood. 2012;119:2579–89.
Goel A, Dispenzieri A, Geyer SM, Greiner S, Peng KW, Russell SJ. Synergistic activity of the proteasome inhibitor PS-341 with non-myeloablative 153-Sm-EDTMP skeletally targeted radiotherapy in an orthotopic model of multiple myeloma. Blood. 2006;107:4063–70.
Janku F, Yap TA, Meric-Bernstam F. Targeting the PI3K pathway in cancer: are we making headway? Nature reviews. Clin Oncol. 2018;15:273–91.
Flinn IW, O’Brien S, Kahl B, Patel M, Oki Y, Foss FF, et al. Duvelisib, a novel oral dual inhibitor of PI3K-delta,gamma, is clinically active in advanced hematologic malignancies. Blood. 2018;131:877–87.
O’Connell MP, Fiori JL, Xu M, Carter AD, Frank BP, Camilli TC, et al. The orphan tyrosine kinase receptor, ROR2, mediates Wnt5A signaling in metastatic melanoma. Oncogene. 2010;29:34–44.
Huang Y, Liu G, Zhang B, Xu G, Xiong W, Yang H. Wnt-5a regulates proliferation in lung cancer cells. Oncol Rep. 2010;23:177–81.
Kurayoshi M, Oue N, Yamamoto H, Kishida M, Inoue A, Asahara T, et al. Expression of Wnt-5a is correlated with aggressiveness of gastric cancer by stimulating cell migration and invasion. Cancer Res. 2006;66:10439–48.
Qiang YW, Walsh K, Yao L, Kedei N, Blumberg PM, Rubin JS, et al. Wnts induce migration and invasion of myeloma plasma cells. Blood. 2005;106:1786–93.
Bolzoni M, Donofrio G, Storti P, Guasco D, Toscani D, Lazzaretti M, et al. Myeloma cells inhibit non-canonical wnt co-receptor ror2 expression in human bone marrow osteoprogenitor cells: effect of wnt5a/ror2 pathway activation on the osteogenic differentiation impairment induced by myeloma cells. Leukemia. 2013;27:451–63.
Hoellenriegel J, Meadows SA, Sivina M, Wierda WG, Kantarjian H, Keating MJ, et al. The phosphoinositide 3’-kinase delta inhibitor, CAL-101, inhibits B-cell receptor signaling and chemokine networks in chronic lymphocytic leukemia. Blood. 2011;118:3603–12.
Piddock RE, Loughran N, Marlein CR, Robinson SD, Edwards DR, Yu S, et al. PI3Kdelta and PI3Kgamma isoforms have distinct functions in regulating pro-tumoural signalling in the multiple myeloma microenvironment. Blood Cancer J. 2017;7:e539.
Anastas JN, Kulikauskas RM, Tamir T, Rizos H, Long GV, von Euw EM, et al. WNT5A enhances resistance of melanoma cells to targeted BRAF inhibitors. J Clin Investig. 2014;124:2877–90.
Steinbrunn T, Stuhmer T, Sayehli C, Chatterjee M, Einsele H, Bargou RC. Combined targeting of MEK/MAPK and PI3K/Akt signalling in multiple myeloma. Br J Haematol. 2012;159:430–40.
Pene F, Claessens YE, Muller O, Viguie F, Mayeux P, Dreyfus F, et al. Role of the phosphatidylinositol 3-kinase/Akt and mTOR/P70S6-kinase pathways in the proliferation and apoptosis in multiple myeloma. Oncogene. 2002;21:6587–97.
Liu J, Zhang Y, Xu R, Du J, Hu Z, Yang L, et al. PI3K/Akt-dependent phosphorylation of GSK3beta and activation of RhoA regulate Wnt5a-induced gastric cancer cell migration. Cell Signal. 2013;25:447–56.
Zhang A, Yan T, Wang K, Huang Z, Liu J. PI3Kalpha isoform-dependent activation of RhoA regulates Wnt5a-induced osteosarcoma cell migration. Cancer Cell Int. 2017;17:27.
Zhang A, He S, Sun X, Ding L, Bao X, Wang N. Wnt5a promotes migration of human osteosarcoma cells by triggering a phosphatidylinositol-3 kinase/Akt signals. Cancer Cell Int. 2014;14:15.
Kawasaki A, Torii K, Yamashita Y, Nishizawa K, Kanekura K, Katada M, et al. Wnt5a promotes adhesion of human dermal fibroblasts by triggering a phosphatidylinositol-3 kinase/Akt signal. Cell Signal. 2007;19:2498–506.
Dai B, Yan T, Zhang A. ROR2 receptor promotes the migration of osteosarcoma cells in response to Wnt5a. Cancer Cell Int. 2017;17:112.
Brown JR. How I treat CLL patients with ibrutinib. Blood . 2018;131:379–86.
Burger JA, Tedeschi A, Barr PM, Robak T, Owen C, Ghia P, et al. Ibrutinib as initial therapy for patients with chronic lymphocytic leukemia. New Engl J Med. 2015;373:2425–37.
Burger JA. Targeting the microenvironment in chronic lymphocytic leukemia is changing the therapeutic landscape. Curr Opin Oncol. 2012;24:643–9.
Pal Singh S, Dammeijer F, Hendriks RW. Role of Bruton’s tyrosine kinase in B cells and malignancies. Mol Cancer. 2018;17:57.
Acknowledgements
The authors wish to thank all the members of Tonon’s lab for collaborative and helpful discussion and critical reading of the paper, and R. Molteni and R. Pardi for helpful suggestions. Funding for this research was provided by Fondazione Cariplo, Association for International Cancer Research (AICR no. 09-0713 to GT), Associazione Italiana per la Ricerca sul Cancro (AIRC Special Program Molecular Clinical Oncology, 5 per mille no. 9965 to GT), Multiple Myeloma Research Foundation (MMRF Research Fellow Award to MF), and Ministero della salute (GR-2011-02351686 to MF).
Author information
Authors and Affiliations
Contributions
MF designed the study, performed experiments, analyzed the data, and wrote the paper. NC, EA, FS, and VV performed experiments. SB and MO helped with in vivo experiments. MG provided technical help and helpful discussion. DB and EF performed 3D experiments. AS assisted with the in vivo imaging. LB and MP performed and analyzed histology samples. SC and RK prepared reagents. PB and FMB performed gene expression profiling. MM provided primary samples. RAD designed the study and critically reviewed the paper. GT designed the study, analyzed the data, and wrote the paper.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest. Although not directly impacting this study, R.A.D. is a co-founder, advisor and director of Tvardi Therapeutics.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Frenquelli, M., Caridi, N., Antonini, E. et al. The WNT receptor ROR2 drives the interaction of multiple myeloma cells with the microenvironment through AKT activation. Leukemia 34, 257–270 (2020). https://doi.org/10.1038/s41375-019-0486-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41375-019-0486-9
This article is cited by
-
Overexpression of Wnt5a promoted the protective effect of mesenchymal stem cells on Lipopolysaccharide-induced endothelial cell injury via activating PI3K/AKT signaling pathway
BMC Infectious Diseases (2024)
-
miR-124-3p and miR-194-5p regulation of the PI3K/AKT pathway via ROR2 in medulloblastoma progression
Cancer Gene Therapy (2024)
-
New insights into the molecular mechanisms of ROR1, ROR2, and PTK7 signaling from the proteomics and pharmacological modulation of ROR1 interactome
Cellular and Molecular Life Sciences (2022)
-
ROR2 has a protective role in melanoma by inhibiting Akt activity, cell-cycle progression, and proliferation
Journal of Biomedical Science (2021)
-
A review on tumor heterogeneity and evolution in multiple myeloma: pathological, radiological, molecular genetics, and clinical integration
Virchows Archiv (2020)