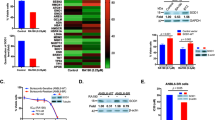
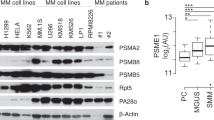
Abstract
Proteasome inhibition is an effective treatment for multiple myeloma (MM); however, targeting different components of the ubiquitin–proteasome system (UPS) remains elusive. Our RNA-interference studies identified proteasome-associated ubiquitin-receptor Rpn13 as a mediator of MM cell growth and survival. Here, we developed the first degrader of Rpn13, WL40, using a small-molecule-induced targeted protein degradation strategy to selectively degrade this component of the UPS. WL40 was synthesized by linking the Rpn13 covalent inhibitor RA190 with the cereblon (CRBN) binding ligand thalidomide. We show that WL40 binds to both Rpn13 and CRBN and triggers degradation of cellular Rpn13, and is therefore first-in-class in exploiting a covalent inhibitor for the development of degraders. Biochemical and cellular studies show that WL40-induced Rpn13 degradation is both CRBN E3 ligase- and Rpn13-dependent. Importantly, WL40 decreases viability in MM cell lines and patient MM cells, even those resistant to bortezomib. Mechanistically, WL40 interrupts Rpn13 function and activates caspase apoptotic cascade, ER stress response and p53/p21 signaling. In animal model studies, WL40 inhibits xenografted human MM cell growth and prolongs survival. Overall, our data show the development of the first UbR Rpn13 degrader with potent anti-MM activity, and provide proof of principle for the development of degraders targeting components of the UPS for therapeutic application.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Kane RC, Bross PF, Farrell AT, Pazdur R. Velcade: U.S. FDA approval for the treatment of multiple myeloma progressing on prior therapy. Oncologist. 2003;8:508–13.
Richardson PG, Barlogie B, Berenson J, Singhal S, Jagannath S, Irwin D, et al. A phase 2 study of bortezomib in relapsed, refractory myeloma. N Engl J Med. 2003;348:2609–17.
Anderson KC. Therapeutic advances in relapsed or refractory multiple myeloma. J Natl Compr Cancer Netw. 2013;11(5 Suppl):676–9.
Richardson PG, Zweegman S, O’Donnell EK, Laubach JP, Raje N, Voorhees P, et al. Ixazomib for the treatment of multiple myeloma. Expert Opin Pharmacother. 2018;19:1949–68.
Lonial S, Waller EK, Richardson PG, Jagannath S, Orlowski RZ, Giver CR, et al. Risk factors and kinetics of thrombocytopenia associated with bortezomib for relapsed, refractory multiple myeloma. Blood. 2005;106:3777–84.
Adams J. The proteasome: a suitable antineoplastic target. Nat Rev Cancer. 2004;4:349–60.
Goldberg AL. Protein degradation and protection against misfolded or damaged proteins. Nature. 2003;426:895–9.
Hershko A. The ubiquitin system for protein degradation and some of its roles in the control of the cell division cycle. Cell Death Differ. 2005;12:1191–7.
Chauhan D, Hideshima T, Anderson KC. Proteasome inhibition in multiple myeloma: therapeutic implication. Annu Rev Pharmacol Toxicol. 2005;45:465–76.
Song Y, Ray A, Li S, Das DS, Tai YT, Carrasco RD, et al. Targeting proteasome ubiquitin receptor Rpn13 in multiple myeloma. Leukemia. 2016;30:1877–86.
Anchoori RK, Karanam B, Peng S, Wang JW, Jiang R, Tanno T, et al. A bis-benzylidine piperidone targeting proteasome ubiquitin receptor RPN13/ADRM1 as a therapy for cancer. Cancer Cell. 2013;24:791–805.
Chen W, Hu XT, Shi QL, Zhang FB, He C. Knockdown of the novel proteasome subunit Adrm1 located on the 20q13 amplicon inhibits colorectal cancer cell migration, survival and tumorigenicity. Oncol Rep. 2009;21:531–7.
Trader DJ, Simanski S, Kodadek T. A reversible and highly selective inhibitor of the proteasomal ubiquitin receptor rpn13 is toxic to multiple myeloma cells. J Am Chem Soc. 2015;137:6312–9.
Fejzo MS, Dering J, Ginther C, Anderson L, Ramos L, Walsh C, et al. Comprehensive analysis of 20q13 genes in ovarian cancer identifies ADRM1 as amplification target. Genes Chromosomes Cancer. 2008;47:873–83.
Husnjak K, Dikic I. Ubiquitin-binding proteins: decoders of ubiquitin-mediated cellular functions. Annu Rev Biochem. 2012;81:291–322.
Schreiner P, Chen X, Husnjak K, Randles L, Zhang N, Elsasser S, et al. Ubiquitin docking at the proteasome through a novel pleckstrin-homology domain interaction. Nature. 2008;453:548–52.
Lu X, Nowicka U, Sridharan V, Liu F, Randles L, Hymel D, et al. Structure of the Rpn13-Rpn2 complex provides insights for Rpn13 and Uch37 as anticancer targets. Nat Commun. 2017;8:15540.
Fejzo MS, Anderson L, Chen HW, Anghel A, Zhuo J, Anchoori R, et al. ADRM1-amplified metastasis gene in gastric cancer. Genes Chromosomes Cancer. 2015;54:506–15.
Carvalho B, Postma C, Mongera S, Hopmans E, Diskin S, van de Wiel MA, et al. Multiple putative oncogenes at the chromosome 20q amplicon contribute to colorectal adenoma to carcinoma progression. Gut. 2009;58:79–89.
Chen X, Walters KJ. Structural plasticity allows UCH37 to be primed by RPN13 or locked down by INO80G. Mol Cell. 2015;57:767–8.
Anchoori RK, Jiang R, Peng S, Soong RS, Algethami A, Rudek MA, et al. Covalent Rpn13-binding inhibitors for the treatment of ovarian cancer. ACS Omega. 2018;3:11917–29.
Cromm PM, Crews CM. Targeted protein degradation: from chemical biology to drug discovery. Cell Chem Biol. 2017;24:1181–90.
Burslem GM, Smith BE, Lai AC, Jaime-Figueroa S, McQuaid DC, Bondeson DP, et al. The advantages of targeted protein degradation over inhibition: an RTK case study. Cell Chem Biol. 2018;25:67–77.e3.
Gustafson JL, Neklesa TK, Cox CS, Roth AG, Buckley DL, Tae HS, et al. Small-molecule-mediated degradation of the androgen receptor through hydrophobic tagging. Angew Chem. 2015;54:9659–62.
Lu J, Qian Y, Altieri M, Dong H, Wang J, Raina K, et al. Hijacking the E3 ubiquitin ligase cereblon to efficiently target BRD4. Chem Biol. 2015;22:755–63.
Sakamoto KM, Kim KB, Kumagai A, Mercurio F, Crews CM, Deshaies RJ. Protacs: chimeric molecules that target proteins to the Skp1-Cullin-F box complex for ubiquitination and degradation. Proc Natl Acad Sci USA. 2001;98:8554–9.
Winter GE, Buckley DL, Paulk J, Roberts JM, Souza A, Dhe-Paganon S, et al. DRUG DEVELOPMENT. Phthalimide conjugation as a strategy for in vivo target protein degradation. Science. 2015;348:1376–81.
Fischer ES, Park E, Eck MJ, Thoma NH. SPLINTS: small-molecule protein ligand interface stabilizers. Curr Opin Struct Biol. 2016;37:115–22.
Raina K, Crews CM. Targeted protein knockdown using small molecule degraders. Curr Opin Chem Biol. 2017;39:46–53.
Toure M, Crews CM. Small-molecule PROTACS: new approaches to protein degradation. Angew Chem. 2016;55:1966–73.
Nowak RP, DeAngelo SL, Buckley D, He Z, Donovan KA, An J. et al. Plasticity in binding confers selectivity in ligand-induced protein degradation. Nat Chem Biol. 2018;14:706–14.
Chauhan D, Singh AV, Brahmandam M, Carrasco R, Bandi M, Hideshima T, et al. Functional interaction of plasmacytoid dendritic cells with multiple myeloma cells: a therapeutic target. Cancer Cell. 2009;16:309–23.
Chauhan D, Catley L, Li G, Podar K, Hideshima T, Velankar M, et al. A novel orally active proteasome inhibitor induces apoptosis in multiple myeloma cells with mechanisms distinct from bortezomib. Cancer Cell. 2005;8:407–19.
Chauhan D, Tian Z, Nicholson B, Kumar KG, Zhou B, Carrasco R, et al. A small molecule inhibitor of ubiquitin-specific protease-7 induces apoptosis in multiple myeloma cells and overcomes bortezomib resistance. Cancer Cell. 2012;22:345–58.
Chauhan D, Ray A, Viktorsson K, Spira J, Paba-Prada C, Munshi N, et al. In vitro and in vivo antitumor activity of a novel alkylating agent, melphalan-flufenamide, against multiple myeloma cells. Clin Cancer Res. 2013;19:3019–31.
Tian Z, Zhao JJ, Tai YT, Amin SB, Hu Y, Berger AJ, et al. Investigational agent MLN9708/2238 targets tumor-suppressor miR33b in MM cells. Blood. 2012;120:3958–67.
Ito T, Ando H, Suzuki T, Ogura T, Hotta K, Imamura Y, et al. Identification of a primary target of thalidomide teratogenicity. Science. 2010;327:1345–50.
Lu G, Middleton RE, Sun H, Naniong M, Ott CJ, Mitsiades CS, et al. The myeloma drug lenalidomide promotes the cereblon-dependent destruction of Ikaros proteins. Science. 2014;343:305–9.
Menendez-Benito V, Verhoef LG, Masucci MG, Dantuma NP. Endoplasmic reticulum stress compromises the ubiquitin-proteasome system. Hum Mol Genet. 2005;14:2787–99.
Bravo R, Parra V, Gatica D, Rodriguez AE, Torrealba N, Paredes F, et al. Endoplasmic reticulum and the unfolded protein response: dynamics and metabolic integration. Int Rev Cell Mol Biol. 2013;301:215–90.
Chauhan D, Hideshima T, Mitsiades C, Richardson P, Anderson KC. Proteasome inhibitor therapy in multiple myeloma. Mol Cancer Ther. 2005;4:686–92.
Chauhan D, Uchiyama H, Akbarali Y, Urashima M, Yamamoto K, Libermann TA, et al. Multiple myeloma cell adhesion-induced interleukin-6 expression in bone marrow stromal cells involves activation of NF-kappa B. Blood. 1996;87:1104–12.
Acknowledgments
The grant support for this investigation was provided by the National Institutes of Health Specialized Programs of Research Excellence (SPORE) grants P50100707, R01CA207237, and RO1CA050947. KCA is an American Cancer Society Clinical Research Professor.
Author information
Authors and Affiliations
Contributions
DC conceptualized the project, designed and supervised all the research, analyzed the data, and wrote the manuscript; YS performed the majority of experiments, generated CRSPR-Cas9 Rpn13-knockout cells, and analyzed the data; LW performed molecule synthesis of WL40; PMCP designed and performed AlphaScreen assays for the CRBN and RPN13 and analyzed the data; AR performed flow cytometry and SCID mouse studies; TD carried out western blotting; SP and PF purified RPN13 protein. VKW and DL reviewed the manuscript. JQ designed biochemical experiments and molecule, and reviewed the manuscript; KCA provided clinical samples, reviewed the data, and wrote the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
KCA is on Advisory board of Celgene, Millenium-Takeda, Gilead, Janssen, and Bristol Myers Squibb, and is a Scientific Founder of Oncopep and C4 Therapeutics. DC is consultant to Stemline Therapeutic, Inc., and Equity owner in C4 Therapeutics. All the remaining authors declare no conflict of interest.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Song, Y., Park, P.M.C., Wu, L. et al. Development and preclinical validation of a novel covalent ubiquitin receptor Rpn13 degrader in multiple myeloma. Leukemia 33, 2685–2694 (2019). https://doi.org/10.1038/s41375-019-0467-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41375-019-0467-z
This article is cited by
-
Proteolysis-targeting chimeras (PROTACs) in cancer therapy
Molecular Cancer (2022)
-
The PROTACtable genome
Nature Reviews Drug Discovery (2021)
-
Advancing targeted protein degradation for cancer therapy
Nature Reviews Cancer (2021)
-
Structure-guided bifunctional molecules hit a DEUBAD-lacking hRpn13 species upregulated in multiple myeloma
Nature Communications (2021)
-
Redirecting proteoxicity
Leukemia (2020)