Abstract
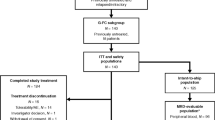
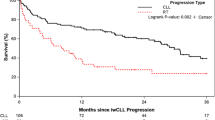
Obinutuzumab (GA101) and ibrutinib show excellent efficacy for treatment of chronic lymphocytic leukemia (CLL). Preclinical investigations and a complementary safety profile were in support of testing their combined use. The exploratory CLL2-BIG-trial evaluated a sequential combination therapy following a recently proposed strategy. Two courses of bendamustine were used for debulking in patients with a high tumor load, followed by six courses of induction therapy with ibrutinib and GA101, followed by an MRD-triggered maintenance phase. The results of a pre-planned analysis at the end of the induction phase are presented. 61 patients were included, 30 previously untreated and 31 with relapsed/refractory CLL. 44 patients received bendamustine. During induction, neutropenia (14.8%) and thrombocytopenia (13.1%) were the most common CTC grade 3 and 4 events. One fatality (duodenitis) occurred. The overall response rate was 100%. 54.1% of patients achieved a partial remission, 41% a clinical complete remission (cCR) without confirmation by CT scan or bone marrow (BM) biopsy according to protocol and 4.9% a cCR with incomplete recovery of the BM. 29 patients (47.5%) had no detectable (<10−4) minimal residual disease assessed by flow cytometry in peripheral blood. In conclusion, the BIG regimen is a safe and highly effective therapy for CLL.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
von Tresckow J, Cramer P, Bahlo J, Engelke A, Langerbeins P, Fink A-M, et al. CLL2-BIG—a novel treatment regimen of bendamustine followed by GA101 and ibrutinib followed by ibrutinib and GA101 maintenance in patients with chronic lymphocytic leukemia (CLL): interim results of a phase II-trial. Blood. 2015;126:4151.
von Tresckow J, Cramer P, Bahlo J, Robrecht S, Engelke A, Langerbeins P, et al. CLL2-BIG—a novel treatment regimen of bendamustine followed by GA101 and ibrutinib followed by ibrutinib and GA101 maintenance in patients with chronic lymphocytic leukemia (CLL): results of a phase II-trial. Blood. 2016;128:640.
Hillmen P, Gribben JG, Follows GA, Milligan D, Sayala HA, Moreton P, et al. Rituximab plus chlorambucil as first-line treatment for chronic lymphocytic leukemia: Final analysis of an open-label phase II study. J Clin Oncol. 2014;32:1236–41.
Goede V, Fischer K, Busch R, Engelke A, Eichhorst B, Wendtner CM, et al. Obinutuzumab plus chlorambucil in patients with CLL and coexisting conditions. N Engl J Med. 2014;370:1101–10.
Foa R, Del Giudice I, Cuneo A, Del Poeta G, Ciolli S, Di Raimondo F, et al. Chlorambucil plus rituximab with or without maintenance rituximab as first-line treatment for elderly chronic lymphocytic leukemia patients. Am J Hematol. 2014;89:480–6.
Eichhorst B, Fink A-M, Bahlo J, Busch R, Kovacs G, Maurer C, et al. First-line chemoimmunotherapy with bendamustine and rituximab versus fludarabine, cyclophosphamide, and rituximab in patients with advanced chronic lymphocytic leukaemia (CLL10): an international, open-label, randomised, phase 3, non-inferiority trial. Lancet Oncol. 2016;17:928–42.
Fischer K, Bahlo J, Fink AM, Goede V, Herling CD, Cramer P, et al. Long-term remissions after FCR chemoimmunotherapy in previously untreated patients with CLL: updated results of the CLL8 trial. Blood. 2016;127:208–15.
Cramer P, von Tresckow J, Bahlo J, Engelke A, Langerbeins P, Fink AM, et al. CLL2-BXX Phase II trials: sequential, targeted treatment for eradication of minimal residual disease in chronic lymphocytic leukemia. Future Oncol. 2018;14:499–513.
Byrd JC, Furman RR, Coutre SE, Flinn IW, Burger JA, Blum KA, et al. Targeting BTK with ibrutinib in relapsed chronic lymphocytic leukemia. N Engl J Med. 2013;369:32–42.
Burger JA, Keating MJ, Wierda WG, Hartmann E, Hoellenriegel J, Rosin NY, et al. Safety and activity of ibrutinib plus rituximab for patients with high-risk chronic lymphocytic leukaemia: a single-arm, phase 2 study. Lancet Oncol. 2014;15:1090–9.
Ysebaert L, Klein C, Quillet-Mary A. CLL cells from ibrutinib-induced lymphocytosis of relapsed/refractory chronic lymphocytic leukemia patients are responsive to obinutuzumab, but not rituximab, ex vivo. Blood. 2015;126:4157.
Hallek M. Signaling the end of chronic lymphocytic leukemia?: new frontline treatment strategies. Blood. 2013;122:3723–34.
Hallek M, Cheson BD, Catovsky D, Caligaris-Cappio F, Dighiero G, Dohner H, et al. Guidelines for the diagnosis and treatment of chronic lymphocytic leukemia: a report from the International Workshop on Chronic Lymphocytic Leukemia updating the National Cancer Institute-Working Group 1996 guidelines. Blood. 2008;111:5446–56.
Böttcher S, Ritgen M, Fischer K, Stilgenbauer S, Busch RM, Fingerle-Rowson G, et al. Minimal residual disease quantification is an independent predictor of progression-free and overall survival in chronic lymphocytic leukemia: a multivariate analysis from the randomized GCLLSG CLL8 trial. J Clin Oncol. 2012;30:980–8.
Fischer K, Cramer P, Busch R, Stilgenbauer S, Bahlo J, Schweighofer CD, et al. Bendamustine combined with rituximab in patients with relapsed and/or refractory chronic lymphocytic leukemia: a multicenter phase II trial of the German Chronic Lymphocytic Leukemia Study Group. J Clin Oncol. 2011;29:3559–66.
Fischer K, Cramer P, Busch R, Bottcher S, Bahlo J, Schubert J, et al. Bendamustine in combination with rituximab for previously untreated patients with chronic lymphocytic leukemia: a multicenter phase II trial of the German Chronic Lymphocytic Leukemia Study Group. J Clin Oncol. 2012;30:3209–16.
Bergmann M, Goebeler M, Herold M, Emmerich B, Wilhelm M, Ruelfs C, et al. Efficacy of bendamustine in patients with relapsed or refractory chronic lymphocytic leukemia: results of a phase I/II study of the German CLL Study Group. Haematologica. 2005;90:1357–64.
Knauf WU, Lissitchkov T, Aldaoud A, Liberati AM, Loscertales J, Herbrecht R, et al. Bendamustine compared with chlorambucil in previously untreated patients with chronic lymphocytic leukaemia: updated results of a randomized phase III trial. Br J Haematol. 2012;159:67–77.
Burger JA, Tedeschi A, Barr PM, Robak T, Owen C, Ghia P, et al. Ibrutinib as initial therapy for patients with chronic lymphocytic leukemia. New Engl J Med. 2015;373:2425–37.
Kovacs G, Robrecht S, Fink AM, Bahlo J, Cramer P, Tresckow Jv, et al. Minimal residual disease assessment improves prediction of outcome in patients with chronic lymphocytic leukemia (CLL) who achieve partial response: comprehensive analysis of two phase III studies of the German CLL Study Group. J Clin Oncol. 2016;34:3758–65.
Byrd JC, Furman RR, Coutre SE, Burger JA, Blum KA, Coleman M, et al. Three-year follow-up of treatment-naïve and previously treated patients with CLL and SLL receiving single-agent ibrutinib. Blood. 2015;125:2497–506.
Acknowledgements
This study was initiated and organized by the German CLL Study Group as an academic trial with the University of Cologne being the sponsor, and financial support by F. Hoffmann La Roche and Janssen-Cilag. The authors wish to express their gratitude towards all patients participating in the trial and their families, as well as the physicians and trial staff at the sites. Furthermore, we thank all study team members involved; in particular, we acknowledge Johanna Wesselmann and Irene Stodden for their excellent contribution. Finally, we thank Dr. Birgit Fath and the monitors from the competence network malignant lymphoma (Kompetenznetz Maligne Lymphome) for facilitating the conduct of this trial.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
JvT: Research funding (F. Hoffmann-LaRoche, Janssen-Cilag), honoraria and consultant or advisory board member (AbbVie, Janssen-Cilag and F. Hoffmann-LaRoche), travel grants (AbbVie, Celgene, F. Hoffmann-LaRoche and Janssen-Cilag). PC: Research funding (AbbVie, Gilead, GlaxoSmithKline/Novartis, F. Hoffmann-LaRoche, and Janssen-Cilag), honoraria (F. Hoffmann-LaRoche and Janssen-Cilag), consultant or advisory board member (AbbVie, AstraZeneca, Janssen-Cilag and Novartis) and travel grants (Astellas, F. Hoffmann LaRoche, Gilead, Janssen-Cilag and Mundipharma). JB: Honoraria and travel grants (F. Hoffmann-LaRoche). PL: Research funding (Janssen-Cilag), Honoraria (AbbVie, Janssen-Cilag and F. Hoffmann-LaRoche), travel grants (AbbVie, F. Hoffmann-LaRoche, Janssen-Cilag and Mundipharma) AF: Research funding (Celgene), honoraria (F. Hoffman-LaRoche) and travel grants (F. Hoffmann-LaRoche, Mundipharma, Abbvie and Celgene). OA: Honoraria and consultant or advisory board member (AbbVie, Gilead and F. Hoffmann-LaRoche), travel grants (AbbVie, Gilead, F. Hoffmann-LaRoche and Janssen-Cilag). TI: Travel grants (F. Hoffmann-LaRoche). MR: Research funding (AbbVie; F. Hoffmann-LaRoche), consultant or advisory board member (AbbVie, BMS, F. Hoffmann-LaRoche) and travel grants (F. Hoffmann-LaRoche). KF: Travels grants (F. Hoffmann-LaRoche). CMW: Honoraria, consultant or advisory board member, research funding and travel grants (AbbVie, Gilead, F. Hoffmann-LaRoche, Janssen-Cilag and Mundipharma). KK: Research funding, honoraria, consultant or advisory board member and speaker´s bureau (F. Hoffmann-LaRoche, Janssen-Cilag and Mundipharma). SS: Research support, honoraria, consultant or advisory board member, speaker´s bureau and travel support (AbbVie, Amgen, Astra-Zeneca, Celgene, F. Hoffmann-LaRoche, Gilead, GlaxoSmithKline, Janssen-Cilag and Novartis). SB: Reasearch funding (AbbVie, Celgene and F. Hoffmann-LaRoche), honoraria (AbbVie, Becton Dickinson, F. Hoffmann-LaRoche), consultant or advisory board member (F. Hoffmann-LaRoche), travel grants (Celgene, F. Hoffmann-LaRoche). BE: Research support, consultant or advisory board member and travel support (AbbVie, Celgene, F. Hoffmann-LaRoche, Gilead, GlaxoSmithKline, Janssen-Cilag, Mundipharma). MH: Research funding (AbbVie, Celgene, F. Hoffmann-LaRoche, Gilead, Janssen-Cilag, Mundipharma and Pharmacyclics), honoraria and speaker´s bureau (AbbVie, Boehringer-Ingelheim, Celgene, F. Hoffmann-LaRoche, Gilead, Janssen-Cilag, Mundipharma and Pharmacyclics) and consultant or advisory board member (AbbVie, F. Hoffmann-LaRoche, Gilead, Janssen-Cilag). The remaining authors declare that they have no conflict of interest.
Supplementary information
Rights and permissions
About this article
Cite this article
von Tresckow, J., Cramer, P., Bahlo, J. et al. CLL2-BIG: sequential treatment with bendamustine, ibrutinib and obinutuzumab (GA101) in chronic lymphocytic leukemia. Leukemia 33, 1161–1172 (2019). https://doi.org/10.1038/s41375-018-0313-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41375-018-0313-8
This article is cited by
-
Chronic Lymphocytic Leukemia: Time-Limited Therapy in the First-Line Setting and Role of Minimal Residual Disease
Current Oncology Reports (2024)
-
Sequential treatment with bendamustine, obinutuzumab (GA101) and Ibrutinib in chronic lymphocytic leukemia (CLL): final results of the CLL2-BIG trial
Leukemia (2022)
-
Richter transformation in chronic lymphocytic leukemia (CLL)—a pooled analysis of German CLL Study Group (GCLLSG) front line treatment trials
Leukemia (2021)
-
Prognostic value of high-sensitivity measurable residual disease assessment after front-line chemoimmunotherapy in chronic lymphocytic leukemia
Leukemia (2021)
-
Is There a Role for Chemotherapy in the Era of Targeted Therapies?
Current Hematologic Malignancy Reports (2020)