Abstract
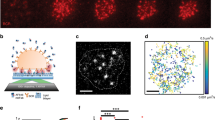
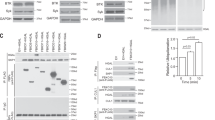
Tonic or chronic active B-cell receptor (BCR) signaling is essential for the survival of normal or some malignant B cells, respectively. However, the molecular mechanism regulating the strength of these two types of BCR signaling remains unknown. Here, using high-speed high-resolution single-molecule tracking in live cells, we identified that PKCβ, STIM1, and IP3R1/2/3 molecules affected the lateral Brownian mobile behavior of BCRs on the plasma membrane of quiescent B cells, which was correlated to the strength of BCR signaling. Further mechanistic studies revealed that these three molecules influenced BCR mobility by regulating the membrane tethering of MARCKS to the inner leaflet of the plasma membrane. Indeed, membrane-untethered or deficiency of MARCKS significantly decreased, while membrane-tethered or overexpression of MARCKS drastically increased the lateral mobility of BCRs. Functional experiments indicated that the membrane-tethered MARCKS suppressed the survival and/or proliferation in both B-cell tumor cells and mouse primary splenic B cells in vitro and in vivo. Mechanistically, we found that membrane-tethered MARCKS increased BCR lateral mobility, and thus decreased BCR nanoclustering by disturbing the interaction between cortical F-actin and the inner leaflet of the plasma membrane, resulting in the suppression of the strength of both tonic and chronic active BCR signaling. Conclusively, MARCKS is a newly identified molecule regulating the strength of BCR signaling by modulating cytoskeleton and plasma membrane interactions, both in the physiological and pathological conditions, suggesting that MARCKS is a putative target for drug design.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
Change history
01 December 2023
A Correction to this paper has been published: https://doi.org/10.1038/s41375-023-02091-9
References
Lam K-P, Kühn R, Rajewsky K. Invivo ablation of surface immunoglobulin on mature B cells by inducible gene targeting results in rapid cell death. Cell. 1997;90:1073–83.
Kraus M, Alimzhanov MB, Rajewsky N, Rajewsky K. Survival of resting mature B lymphocytes depends on BCR signaling via the Igα/β heterodimer. Cell. 2004;117:787–800.
Schweighoffer E, Vanes L, Nys J, Cantrell D, McCleary S, Smithers N, et al. The BAFF receptor transduces survival signals by co-opting the B cell receptor signaling pathway. Immunity. 2013;38:475–88.
Love PE, Shores EW, Johnson MD, Tremblay ML, Lee EJ, Grinberg A, et al. T cell development in mice that lack the zeta chain of the T cell antigen receptor complex. Science. 1993;261:918.
Wang K, Yamamoto H, Chin JR, Werb Z, Vu TH. Epidermal growth factor receptor-deficient mice have delayed primary endochondral ossification because of defective osteoclast recruitment. J Biol Chem. 2004;279:53848–56.
Frederic M, Antonio F, Stephanie S, Laurence T, Serge L, Francois M-G, et al. A novel immunodeficient mouse model--RAG2 x common cytokine receptor gamma chain double mutants--requiring exogenous cytokine administration for human hematopoietic stem cell engraftment. J Interferon Cytokine Res. 1998;19:533–41.
Baird AM, Gerstein RM, Berg LJ. The role of cytokine receptor signaling in lymphocyte development. Curr Opin Immunol. 1999;11:157–66.
Rajewsky K. Clonal selection and learning in the antibody system. Nature. 1996;381:751–8.
Okkenhaug K, Burger JA. PI3K signaling in normal B cells and chronic lymphocytic leukemia (CLL). In: Kurosaki T, Wienands J, editors. B cell receptor signaling. Springer: Cham, Switzerland, 2015. p. 123–42.
Davis RE, Ngo VN, Lenz G, Tolar P, Young RM, Romesser PB, et al. Chronic active B-cell-receptor signalling in diffuse large B-cell lymphoma. Nature. 2010;463:88–92.
Young RM, Staudt LM. Targeting pathological B cell receptor signalling in lymphoid malignancies. Nat Rev Drug Discov. 2013;12:229–43.
Chen L, Monti S, Juszczynski P, Daley J, Chen W, Witzig TE, et al. SYK-dependent tonic B-cell receptor signaling is a rational treatment target in diffuse large B-cell lymphoma. Blood. 2008;111:2230.
Pore D, Bodo J, Danda A, Yan D, Phillips JG, Lindner D, et al. Identification of Ezrin-Radixin-Moesin proteins as novel regulators of pathogenic B cell receptor signaling and tumor growth in diffuse large B cell lymphoma. Leukemia. 2015;29:1857–67.
Hendriks RW, Yuvaraj S, Kil LP. Targeting Bruton’s tyrosine kinase in B cell malignancies. Nat Rev Cancer. 2014;14:219–32.
Schmitz R, Young RM, Ceribelli M, Jhavar S, Xiao W, Zhang M, et al. Burkitt lymphoma pathogenesis and therapeutic targets from structural and functional genomics. Nature. 2012;490:116–20.
Wang ML, Rule S, Martin P, Goy A, Auer R, Kahl BS, et al. Targeting BTK with Ibrutinib in relapsed or refractory mantle-cell lymphoma. N Engl J Med. 2013;369:507–16.
Naylor TL, Tang H, Ratsch BA, Enns A, Loo A, Chen L, et al. Protein kinase C inhibitor sotrastaurin selectively inhibits the growth of CD79 mutant diffuse large B-cell lymphomas. Cancer Res. 2011;71:2643.
Moreau A-S, Jia X, Ngo HT, Leleu X, Sullivan G, Alsayed Y, et al. Protein kinase C inhibitor enzastaurin induces in vitro and in vivo antitumor activity in Waldenström macroglobulinemia. Blood. 2007;109:4964.
Treanor B, Depoil D, Gonzalez-Granja A, Barral P, Weber M, Dushek O, et al. The membrane skeleton controls diffusion dynamics and signaling through the B cell receptor. Immunity. 2010;32:187–99.
Liu W, Wang H, Xu C. Antigen receptor nanoclusters: small units with big functions. Trends Immunol. 2016;37:680–9.
Mattila Pieta K, Feest C, Depoil D, Treanor B, Montaner B, Otipoby Kevin L, et al. The actin and tetraspanin networks organize receptor nanoclusters to regulate B cell receptor-mediated signaling. Immunity. 2013;38:461–74.
Lee J, Sengupta P, Brzostowski J, Lippincott-Schwartz J, Pierce SK, Lidke D. The nanoscale spatial organization of B-cell receptors on immunoglobulin M– and G–expressing human B-cells. Mol Biol Cell. 2017;28:511–23.
Yang J, Reth M. Oligomeric organization of the B-cell antigen receptor on resting cells. Nature. 2010;467:465–9.
Kusumi A, Fujiwara TK, Chadda R, Xie M, Tsunoyama TA, Kalay Z, et al. Dynamic organizing principles of the plasma membrane that regulate signal transduction: commemorating the fortieth anniversary of Singer and Nicolson’s fluid-mosaic model. Annu Rev Cell Dev Biol. 2012;28:215–50.
Tolar P, Hanna J, Krueger PD, Pierce SK. The constant region of the membrane immunoglobulin mediates B cell-receptor clustering and signaling in response to membrane antigens. Immunity. 2009;30:44–55.
Wang J, Tang S, Wan Z, Gao Y, Cao Y, Yi J, et al. Utilization of a photoactivatable antigen system to examine B-cell probing termination and the B-cell receptor sorting mechanisms during B-cell activation. Proc Natl Acad Sci USA. 2016;113:E558–67.
Liu W, Meckel T, Tolar P, Won Sohn H, Pierce SK. Intrinsic properties of immunoglobulin IgG1 isotype-switched B cell receptors promote microclustering and the initiation of signaling. Immunity. 2010;32:778–89.
Liu W, Meckel T, Tolar P, Sohn HW, Pierce SK. Antigen affinity discrimination is an intrinsic function of the B cell receptor. J Exp Med. 2010;207:1095–111.
Treanor B, Depoil D, Bruckbauer A, Batista FD. Dynamic cortical actin remodeling by ERM proteins controls BCR microcluster organization and integrity. J Exp Med. 2011;208:1055–68.
Yasuda T, Yamamoto T. Analysis of B-cell signaling using DT40 B-cell line. In: Gu H, Rajewsky K, editors. B cell protocols.Totowa, NJ: Humana Press; 2004. p. 261–70. .
Winding P, Berchtold MW. The chicken B cell line DT40: a novel tool for gene disruption experiments. J Immunol Methods. 2001;249:1–16.
Shinohara H, Yasuda T, Aiba Y, Sanjo H, Hamadate M, Watarai H, et al. PKCβ regulates BCR-mediated IKK activation by facilitating the interaction between TAK1 and CARMA1. J Exp Med. 2005;202:1423.
Baba Y, Hayashi K, Fujii Y, Mizushima A, Watarai H, Wakamori M, et al. Coupling of STIM1 to store-operated Ca(2+) entry through its constitutive and inducible movement in the endoplasmic reticulum. Proc Natl Acad Sci USA. 2006;103:16704–9.
Sugawara H, Kurosaki M, Takata M, Kurosaki T. Genetic evidence for involvement of type 1, type 2 and type 3 inositol 1,4,5‐trisphosphate receptors in signal transduction through the B‐cell antigen receptor. EMBO J. 1997;16:3078.
Liu W, Won Sohn H, Tolar P, Meckel T, Pierce SK. Antigen-induced oligomerization of the B cell receptor is an early target of FcγRIIB inhibition. J Immunol. 2010;184:1977–89.
Xu L, Li G, Wang J, Fan Y, Wan Z, Zhang S, et al. Through an ITIM-independent mechanism the FcγRIIB blocks B cell activation by disrupting the colocalized microclustering of the B cell receptor and CD19. J Immunol. 2014;192:5179–91.
Liu W, Chen E, Zhao XW, Wan ZP, Gao YR, Davey A. et al. The scaffolding protein Synapse-Associated Protein 97 is required for enhanced signaling through isotype-switched IgG memory B cell receptors. Sci Signal. 2012;5:ra54
Kurosaki T, Shinohara H, Baba Y. B cell signaling and fate decision. Annu Rev Immunol. 2010;28:21–55.
Kurosaki T. Genetic analysis of B cell antigen receptor signaling. Annu Rev Immunol. 1999;17:555–92.
Arbuzova A, Schmitz AAP, VergÈRes G. Cross-talk unfolded: MARCKS proteins. Biochem J. 2002;362:1–12.
Trovo L, Ahmed T, Callaerts-Vegh Z, Buzzi A, Bagni C, Chuah M, et al. Low hippocampal PI(4,5)P2 contributes to reduced cognition in old mice as a result of loss of MARCKS. Nat Neurosci. 2013;16:449–55.
Gadi D, Wagenknecht-Wiesner A, Holowka D, Baird B. Sequestration of phosphoinositides by mutated MARCKS effector domain inhibits stimulated Ca2+ mobilization and degranulation in mast cells. Mol Biol Cell. 2011;22:4908–17.
McLaughlin S, Murray D. Plasma membrane phosphoinositide organization by protein electrostatics. Nature. 2005;438:605–11.
Chen X, Pan W, Sui Y, Li H, Shi X, Guo X, et al. Acidic phospholipids govern the enhanced activation of IgG-B cell receptor. Nat Commun. 2015;6:8552.
Chu C, Wang Y, Zhang X, Ni X, Cao J, Xu W, et al. SAP-regulated T cell–APC adhesion and ligation-dependent and -independent Ly108–CD3ζ interactions. J Immunol. 2014;193:3860.
Baba Y, Kurosaki T. Impact of Ca2+ signaling on B cell function. Trends Immunol. 2011;32:589–94.
Ziemba Brian P, Burke John E, Masson G, Williams Roger L, Falke Joseph J. Regulation of PI3K by PKC and MARCKS: single-molecule analysis of a reconstituted signaling pathway. Biophys J. 2016;110:1811–25.
Knight JD, Lerner MG, Marcano-Velázquez JG, Pastor RW, Falke Joseph J. Single molecule diffusion of membrane-bound proteins: window into lipid contacts and bilayer dynamics. Biophys J. 2010;99:2879–87.
Knight JD, Falke JJ. Single-molecule fluorescence studies of a PH domain: new insights into the membrane docking reaction. Biophys J. 2009;96:566–82.
Xu L, Xia M, Guo J, Sun X, Li H, Xu C, et al. Impairment on the lateral mobility induced by structural changes underlies the functional deficiency of the lupus-associated polymorphism FcgammaRIIB-T232. J Exp Med. 2016;213:2707–27.
Zhang J, Leiderman K, Pfeiffer JR, Wilson BS, Oliver JM, Steinberg SL. Characterizing the topography of membrane receptors and signaling molecules from spatial patterns obtained using nanometer-scale electron-dense probes and electron microscopy. Micron. 2006;37:14–34.
Gambhir A, Hangyás-Mihályné G, Zaitseva I, Cafiso DS, Wang J, Murray D, et al. Electrostatic sequestration of PIP2 on phospholipid membranes by basic/aromatic regions of proteins. Biophys J. 2004;86:2188–207.
Wang J, Arbuzova A, Hangyas-Mihalyne G, McLaughlin S. The effector domain of myristoylated alanine-rich C kinase substrate binds strongly to phosphatidylinositol 4,5-bisphosphate. J Biol Chem. 2001;276:5012–9.
Wang J, Gambhir A, Hangyas-Mihalyne G, Murray D, Golebiewska U, McLaughlin S. Lateral sequestration of phosphatidylinositol 4,5-bisphosphate by the basic effector domain of myristoylated alanine-rich C kinase substrate is due to nonspecific electrostatic interactions. J Biol Chem. 2002;277:34401–12.
McLaughlin S, Wang J, Gambhir A, Murray D. PIP2 and proteins: interactions, organization, and information flow. Annu Rev Biophys Biomol Struct. 2002;31:151–75.
Yin HL, Janmey PA. Phosphoinositide regulation of the actin cytoskeleton. Annu Rev Physiol. 2003;65:761–89.
Raucher D, Stauffer T, Chen W, Shen K, Guo S, York JD, et al. Phosphatidylinositol 4,5-bisphosphate functions as a second messenger that regulates cytoskeleton–plasma membrane adhesion. Cell. 2000;100:221–8.
Grashoff C, Hoffman BD, Brenner MD, Zhou R, Parsons M, Yang MT, et al. Measuring mechanical tension across vinculin reveals regulation of focal adhesion dynamics. Nature. 2010;466:263–6.
Brenner MD, Zhou R, Conway DE, Lanzano L, Gratton E, Schwartz MA, et al. Spider silk Peptide is a compact, linear nanospring ideal for intracellular tension sensing. Nano Lett. 2016;16:2096–102.
Resh MD. Myristylation and palmitylation of Src family members: the fats of the matter. Cell. 1994;76:411–3.
Suh B-C, Inoue T, Meyer T, Hille B. Rapid chemically induced changes of PtdIns(4,5)P2 gate KCNQ ion channels. Science. 2006;314:1454–7.
Fehon RG, McClatchey AI, Bretscher A. Organizing the cell cortex: the role of ERM proteins. Nat Rev Mol Cell Biol. 2010;11:276–87.
Kusumi A, Nakada C, Ritchie K, Murase K, Suzuki K, Murakoshi H, et al. Paradigm shift of the plasma membrane concept from the two-dimensional continuum fluid to the partitioned fluid: high-speed single-molecule tracking of membrane molecules. Annu Rev Biophys Biomol Struct. 2005;34:351–78.
Kasai RS, Suzuki KGN, Prossnitz ER, Koyama-Honda I, Nakada C, Fujiwara TK, et al. Full characterization of GPCR monomer–dimer dynamic equilibrium by single molecule imaging. J Cell Biol. 2011;192:463.
Hamada K, Shimizu T, Matsui T, Tsukita S, Tsukita S, Hakoshima T. Structural basis of the membrane‐targeting and unmasking mechanisms of the radixin FERM domain. EMBO J. 2000;19:4449–62.
Ben-Aissa K, Patino-Lopez G, Belkina NV, Maniti O, TR, Hao JJ, et al. Activation of moesin, a protein that links actin cytoskeleton to the plasma membrane, occurs by phosphatidylinositol 4,5-bisphosphate (PIP2) binding sequentially to two sites and releasing an autoinhibitory linker. J Biol Chem. 2012;287:16311–23.
Braunger JA, Bruckner BR, Nehls S, Pietuch A, Gerke V, Mey I, et al. Phosphatidylinositol 4,5-bisphosphate alters the number of attachment sites between ezrin and actin filaments: a colloidal probe study. J Biol Chem. 2014;289:9833–43.
Bickeboller M, Tagscherer KE, Kloor M, Jansen L, Chang-Claude J, Brenner H, et al. Functional characterization of the tumor-suppressor MARCKS in colorectal cancer and its association with survival. Oncogene. 2015;34:1150–9.
Michel S, Kloor M, Singh S, Gdynia G, Roth W, von Knebel Doeberitz M, et al. Coding microsatellite instability analysis in microsatellite unstable small intestinal adenocarcinomas identifies MARCKS as a common target of inactivation. Mol Carcinog. 2010;49:175–82.
Kim N-G, Rhee H, Li LS, Kim H, Lee J-S, Kim J-H, et al. Identification of MARCKS, FLJ11383 and TAF1B as putative novel target genes in colorectal carcinomas with microsatellite instability. Oncogene. 2002;21:5081–7.
Jarboe JS, Anderson JC, Duarte CW, Mehta T, Nowsheen S, Hicks PH, et al. MARCKS regulates growth and radiation sensitivity and is a novel prognostic factor for glioma. Clin Cancer Res. 2012;18:3030–41.
Gutierrez NC, Ocio EM, de las Rivas J, Maiso P, Delgado M, Ferminan E, et al. Gene expression profiling of B lymphocytes and plasma cells from Waldenstrom’s macroglobulinemia: comparison with expression patterns of the same cell counterparts from chronic lymphocytic leukemia, multiple myeloma and normal individuals. Leukemia. 2007;21:541–9.
Simek SL, Kligman D, Patel J, Colburn NH. Differential expression of an 80-kDa protein kinase C substrate in preneoplastic and neoplastic mouse JB6 cells. Proc Natl Acad Sci USA. 1989;86:7410–4.
Joseph CK, Qureshi SA, Wallace DJ, Foster DA. MARCKS protein is transcriptionally down-regulated in v-Src-transformed BALB/c 3T3 cells. J Biol Chem. 1992;267:1327–30.
Wolfman A, Wingrove TG, Blackshear PJ, Macara IG. Down-regulation of protein kinase C and of an endogenous 80-kDa substrate in transformed fibroblasts. J Biol Chem. 1987;262:16546–52.
Brooks G, Brooks SF, Goss MW. MARCKS functions as a novel growth suppressor in cells of melanocyte origin. Carcinogenesis. 1996;17:683–9.
Acknowledgements
We thank Dr. Tomohiro Kurosaki and Dr. Hisaaki Shinohara (WPI Immunology Frontier Research Center, Osaka University, Japan) for providing DT40 cell lines, Dr. Carlos G. Dotti (Consejo Superior de Investigaciones Científicas) for providing MARCKS plasmid, Dr. Feng Zhang (MIT, Cambridge) for providing plasmids of pSpCas9-2a-GFP. We are supported by funds from National Science Foundation China (8182500030, 81730043 and 81621002) and Ministry of Science and Technology of China (2014CB542500-03).
Author Contributions
CX, YF and YJ performed the experiments and statistical analysis. CX and WL conceived the project. WL supervised the project. YZ, CL and YZ provided suggestions. CX and WL wrote the manuscript.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Xu, C., Fang, Y., Yang, Z. et al. MARCKS regulates tonic and chronic active B cell receptor signaling. Leukemia 33, 710–729 (2019). https://doi.org/10.1038/s41375-018-0244-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41375-018-0244-4
This article is cited by
-
MCT1-governed pyruvate metabolism is essential for antibody class-switch recombination through H3K27 acetylation
Nature Communications (2024)
-
Pathophysiological roles of myristoylated alanine-rich C-kinase substrate (MARCKS) in hematological malignancies
Biomarker Research (2021)