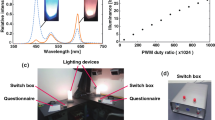
Abstract
Objective
We designed and implemented a novel neonatal intensive care (NICU) lighting system to support the current understanding of daylight-coupled physiology.
Methods
We created a system that generates wavelengths corresponding to the known blue and violet activation spectra of non-visual opsins. These are known to mediate energy management and related physiologic activity.
Results
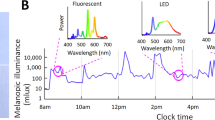
Light produced by the system spans the visible spectrum, including violet wavelengths that are blocked by modern glazing and not emitted by standard LED fixtures. System features include automated light and dark phases that mimic dawn/dusk. The system also matches length of day seasonality. Spectral composition can be varied to support translational research protocols. Implementation required a comprehensive strategy to inform bedside providers about the value and use of the lighting system.
Conclusion
Full-spectrum lighting for the NICU is feasible and will inform the optimization of the NICU environment of care to support optimal neonatal growth and development.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
The datasets generated during and/or analyzed for this article are available from the corresponding author on reasonable request.
References
Webb AR, Heller HT, Benson CB, Lahav A. Mother’s voice and heartbeat sounds elicit auditory plasticity in the human brain before full gestation. Proc Natl Acad Sci USA. 2015;112:3152–7.
Watanabe S, Akiyama S, Hanita T, Li H, Nakagawa M, Kaneshi Y, et al. Designing artificial environments for preterm infants based on circadian studies on pregnant uterus. Front Endocrinol (Lausanne). 2013;4:113.
Vasquez-Ruiz S, Maya-Barrios JA, Torres-Narvaez P, Vega-Martinez BR, Rojas-Granados A, Escobar C, et al. A light/dark cycle in the NICU accelerates body weight gain and shortens time to discharge in preterm infants. Early Hum Dev. 2014;90:535–40.
Brandon DH, Holditch-Davis D, Belyea M. Preterm infants born at less than 31 weeks’ gestation have improved growth in cycled light compared with continuous near darkness. J Pediatr. 2002;140:192–9.
Morag I, Ohlsson A. Cycled light in the intensive care unit for preterm and low birth weight infants. Cochrane Database Syst Rev. 2016;2016:CD006982.
Hu J, Shi Y, Zhang J, Huang X, Wang Q, Zhao H, et al. Melanopsin retinal ganglion cells mediate light-promoted brain development. Cell. 2022;185:3124–37.e15.
Kojima D, Mori S, Torii M, Wada A, Morishita R, Fukada Y. UV-sensitive photoreceptor protein OPN5 in humans and mice. PLoS ONE. 2011;6:e26388.
Nagata T, Koyanagi M, Lucas R, Terakita A. An all-trans-retinal-binding opsin peropsin as a potential dark-active and light-inactivated G protein-coupled receptor. Sci Rep. 2018;8:3535.
Contreras E, Nobleman AP, Robinson PR, Schmidt TM. Melanopsin phototransduction: beyond canonical cascades. J Exp biol. 2021;224:jeb226522.
Nayak G, Zhang KX, Vemaraju S, Odaka Y, Buhr ED, Holt-Jones A, et al. Adaptive thermogenesis in mice is enhanced by opsin 3-dependent adipocyte light sensing. Cell Rep. 2020;30:672–86.e8.
Zhang KX, D’Souza S, Upton BA, Kernodle S, Vemaraju S, Nayak G, et al. Violet-light suppression of thermogenesis by opsin 5 hypothalamic neurons. Nature. 2020;585:420–5.
Upton BA, Diaz NM, Gordon SA, Van Gelder RN, Buhr ED, Lang RA. Evolutionary constraint on visual and nonvisual mammalian opsins. J Biol Rhythms. 2021;36:109–26.
White RD, Smith JA, Shepley MM, Committee to Establish Recommended Standards for Newborn ICUD. Recommended standards for newborn ICU design, eighth edition. J Perinatol. 2013;33:S2–16.
Keunen K, van Elburg RM, van Bel F, Benders MJ. Impact of nutrition on brain development and its neuroprotective implications following preterm birth. Pediatr Res. 2015;77:148–55.
Hazelhoff EM, Dudink J, Meijer JH, Kervezee L. Beginning to see the light: lessons learned from the development of the circadian system for optimizing light conditions in the neonatal intensive care unit. Front Neurosci. 2021;15:634034.
Wilkerson A, Safranek S, Irvin L, Tredinnick L. Lighting system control data to improve design and operation: tunable lighting system data from NICU patient rooms. LEUKOS. 2023;19:94–109.
Rao S, Chun C, Fan J, Kofron JM, Yang MB, Hegde RS, et al. A direct and melanopsin-dependent fetal light response regulates mouse eye development. Nature. 2013;494:243–6.
Jiang X, Pardue MT, Mori K, Ikeda SI, Torii H, D’Souza S, et al. Violet light suppresses lens-induced myopia via neuropsin (OPN5) in mice. Proc natl Acd Sci USA. 2021;118:1–8.
Buhr ED, Vemaraju S, Diaz N, Lang RA, Van Gelder RN. Neuropsin (OPN5) mediates local light-dependent induction of circadian clock genes and circadian photoentrainment in exposed murine skin. Curr Biol. 2019;29:3478–87.e4.
Acknowledgements
We are grateful for the collaboration of Robert Soler (BIOS LLC), Marty Brennan (ZGF Architects), Jeannine Wang (Acuity Brands), Lauri Tredinnick (Pivotal Lighting Design), Greg Goertemoeller (Messer Construction), Michael Browning (Cincinnati Children’s), Oliver Rhine (Cincinnati Children’s), Alex Miller (Cincinnati Children’s), Tony Johnston (Cincinnati Children’s), and Steve Davis (Cincinnati Children’s).
Funding
The NICU spectral lighting system was funded as a component of the Cincinnati Children’s Critical Care Building construction. The Science of Light Center receives funding through the Academic Research Committee of the Cincinnati Children’s Research Foundation. This work was supported in part by funding to RAL from the National Eye Institute of the National Institutes of Health (EY032029, EY032752, and EY032566), from the McClung Foundation, and from the Emma and Irving Goldman Scholar endowed Chair of the Abrahamson Pediatric Eye Institute.
Author information
Authors and Affiliations
Contributions
JMG and RAL conceived, developed, and designed the lighting system and wrote the manuscript. KAG, LR, JNS, DK, and YC developed measurement methods and performed spectral analyses.
Corresponding author
Ethics declarations
Competing interests
Drs. RAL and JMG are co-inventors on a patent licensed to BIOS, LLC. Cincinnati Children’s Hospital has licensed this technology to BIOS, LLC, the designer of the lighting system used in this study. Drs. RAL and JMG do not have any personal financial interest in BIOS, LLC, but could receive royalty payments through Cincinnati Children’s Hospital.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Greenberg, J.M., Gruner, K.A., Rodney, L. et al. Biologically aware lighting for newborn intensive care. J Perinatol 43 (Suppl 1), 49–54 (2023). https://doi.org/10.1038/s41372-023-01816-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41372-023-01816-z