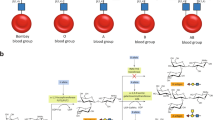
Abstract
Hemolytic disease of the fetus and newborn (HDFN) can occur when a pregnant woman has antibody directed against an erythrocyte surface antigen expressed by her fetus. This alloimmune disorder is restricted to situations where transplacental transfer of maternal antibody to the fetus occurs, and binds to fetal erythrocytes, and significantly shortens the red cell lifespan. The pathogenesis of HDFN involves maternal sensitization to erythrocyte “non-self” antigens (those she does not express). Exposure of a woman to a non-self-erythrocyte antigen principally occurs through either a blood transfusion or a pregnancy where paternally derived erythrocyte antigens, expressed by her fetus, enter her circulation, and are immunologically recognized as foreign. This review focuses on the genetics, structure, and function of the erythrocyte antigens that are most frequently involved in the pathogenesis of alloimmune HDFN. By providing this information we aim to convey useful insights to clinicians caring for patients with this condition.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Kark Landsteiner receivinthe 1930 Nobel Prize in Medicine. https://www.rockefeller.edu/our-scientists/karl-landsteiner/2554-nobel-prize/.
Diamond LK, Blackfan KD, Baty JM. Erythroblastosis fetalis and its association with universal edema of the fetus, icterus gravis neonatorum and anemia of the newborn. J Pediatr. 1932;1:269–309.
Landsteiner K, Wiener AS. An agglutinable factor in human blood recognized by immune sera for Rhesus blood. Proc Soc Exp Biol Med. 1940;43:223.
Mollison PL, Cutbush M. Hemolytic disease of the newborn; criteria of severity. Br Med J. 1949;4594:123–30.
Freda VJ, Gorman JG, Pollack W. Rh factor: prevention of isoimmunization and clinical trial on mothers. Science. 1966;151:828–30.
Freda VJ, Gorman JG, Pollack W, Bowe E. Prevention of Rh hemolytic disease–ten years’ clinical experience with Rh immune globulin. N Engl J Med. 1975;292:1014–6.
Freda VJ, Gorman JG, Pollack W. Prevention of Rh-hemolytic disease with Rh-immune globulin. Am J Ob Gyn. 1977;128:456–60.
Zipursky A, Paul VK. The global burden of Rh disease. Arch Dis Child Fetal Neonatal Ed. 2011;96:F84–5.
Moise KJ. Fetal anemia due to non-Rhesus-D red-cell alloimmunization. Semin Fetal Neonatal Med. 2008;13:207–14.
van Kamp IL, Klumper FJ, Meerman RH, Oepkes D, Scherjon SA, Kanhai HH. Treatment of fetal anemia due to red cell alloimmunization with intrauterine transfusions in the Netherlands, 1988-1999. Acta Obstet Gynecol Scand. 2004;83:731–7.
de Haas M, Thurik FF, Koelewijn JM, van der Schoot CE. Haemolytic disease of the fetus and newborn. Vox Sang. 2015;109:99–113.
Yu D, Ling LE, Krumme AA, Tjoa ML, Moise KJ Jr. Live birth prevalence of hemolytic disease of the fetus and newborn in the United States from 1996 to 2010. AJOG Glob Rep. 2023;3:100203.
Christensen RD, Bahr TM, Wong RJ, Vreman HJ, Bhutani VK, Stevenson DKA “gold standard” test for diagnosing and quantifying hemolysis in neonates and infants. J Perinatol. 2023. https://doi.org/10.1038/s41372-023-01730-4.
Thiagarajan P, Parker CJ, Prchal JT. How do red blood cells die? Front Physiol. 2021;12:65539314. Mar 15
Cooper RA, Shattil SJH. Mechanisms of hemolysis - the minimal red cell defect. N Eng J Med. 1971;285:1514–20.
Quigley JG, Means Jr RT, Glader B The birth, life, and death of red blood cells; erythropoiesis, the mature red blood cell, and cell destruction. In Wintrobe’s Clinical Hematololgy, 14th Edition, Greer JP, Rodgers GM, Glader B, Arber DA, Means Je RT, List AF, Applebaum FR, Dispenziere A, Fehniger TA, eds., Wolters Kluwer, Philadelphia, PA, 2019; pp 116-7.
Urbaniak SJ, Greiss MA. RhD hemolytic disease of the fetus and the newborn. Blood Rev. 2000;14:44–61.
Durand JK, Willis MS. Karl Landsteiner, MD: Transfusion Medicine. Lab Med. 2010;41:53–5.
Chérif-Zahar, B, Mattéi MG, Le Van Kim C. Localization of the human Rh blood group gene structure to chromosome region 1p34.3-1p36.1 by in situ hybridization. Hum Genet. 1991;86:398–400.
Dean L Blood Groups and Red Cell Antigens [Internet]. Bethesda (MD): National Center for Biotechnology Information (US). https://www.ncbi.nlm.nih.gov/books/NBK2275/.
Vallese F, Kim K, Yen LY, Johnston JD, Noble AJ, Calì T, et al. Architecture of the human erythrocyte ankyrin-1 complex. Nat Struct Mol Biol. 2022;29:706–18.
Lieberman L, Callum J, Cohen R, Cserti-Gazdewich C, Ladhani NNN, Buckstein J, et al. Impact of red blood cell alloimmunization on fetal and neonatal outcomes: A single center cohort study. Transfusion. 2020;60:2537–46.
Ree IMC, Besuden CFJ, Wintjens VEHJ, Verweij JEJT, Oepkes D, de Haas M, et al. Exchange transfusions in severe Rh-mediated alloimmune haemolytic disease of the foetus and newborn: a 20-year overview on the incidence, associated risks, and outcome. Vox Sang. 2021;116:990–7.
De Winter DP, Hulzebos C, Van ‘t Oever RM, DeHaas M, Verweij EJT, Lopriore E. History and current standard of postnatal management in hemolytic disease of the fetus and newborn. Eur J Pediatr. 2023;182:489–500.
Hackney DN, Knudtson EJ, Rossi KQ, Krugh D, O’Shaughnessy RW. Management of pregnancies complicated by anti-c isoimmunization. Obstet Gynecol. 2004;103:24–30.
Appelman Z, Lurie S, Juster A, Borenstein R. Severe hemolytic disease of the newborn due to anti-c. Int J Gynaecol Obstet. 1990;33:73–5.
Bowman JM, Pollock J. Maternal CW alloimmunization. Vox Sang. 1993;64:226–30.
Finney RD, Blue AM, Willoughby ML. Haemolytic disease of the newborn caused by the rare Rhesus antibody anti-CX. Vox Sang. 1973;25:39–42.
Bowman JM, Pollock JM, Manning FA, Harman CR. Severe anti-C hemolytic disease of the newborn. Am J Obstet Gynecol. 1992;166:1239–43.
Joy SD, Rossi KQ, Krugh D, O’Shaughnessy RW. Management of pregnancies complicated by anti-E alloimmunization. Obstet Gynecol. 2005;105:24–8.
Chapman J, Waters A. Haemolytic disease of the newborn due to Rhesus anti-e antibody. Vox Sang. 1981;41:45–7.
The ABO blood group - Blood Groups and Red Cell Antigens - NCBI Bookshelf (nih.gov).
Dean L ABO Blood Group. 2012 Oct 1 [updated 2015 Jul 27]. In: Pratt VM, Scott SA, Pirmohamed M, Esquivel B, Kattman BL, Malheiro AJ, editors. Medical Genetics Summaries [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2012.
Scharberg EA, Olsen C, Bugert P. The H blood group system. Immunohematology. 2016;32:112–8.
Bullock T, Win N, Jackson B, Sivarajan S, Penny J, Mir N. Bombay phenotype (Oh) and high-titer anti-H in pregnancy: two case reports and a review of the literature. Transfusion. 2018;58:2766–72.
Storch EK, Rogerson B, Eder AF. Trend in ABO-incompatible RBC transfusion-related fatalities reported to the FDA, 2000-2019. Transfusion 2020;60:2867–75.
Branch DR. Anti-A and anti-B: what are they and where do they come from? Transfusion. 2015;55:S74–9.
Krog GR, Lorenzen H, Clausen FB, Dziegiel MH. Secretor status of blood group O mothers is associated with development of ABO haemolytic disease in the newborn. Vox Sang. 2023;118:402–6.
Watchko JF. ABO hemolytic disease of the newborn: a need for clarity and consistency in diagnosis. J Perinatol. 2023;43:242–7.
Routray SS, Mishra D, Kanungo GN, Behera R. Hemolytic disease of newborn due to ABO incompatibility between B blood group mother and A blood group neonate. J Lab Physicians. 2022;15:146–8.
Christensen RD, Baer VL, MacQueen BC, O’Brien EA, Ilstrup SJ. ABO hemolytic disease of the fetus and newborn: thirteen years of data after implementing a universal bilirubin screening and management program. J Perinatol. 2018;38:517–25.
Baer VL, Hulse W, Bahr TM, Ilstrup SJ, Christensen RD. Absence of severe neonatal ABO hemolytic disease at Intermountain Healthcare. Why? J Perinatol. 2020;40:352–3.
Miller DF, Petrie SJ. Fatal erythroblastosis fetalis secondary to ABO incompatibility. Report of a case. Obstet Gynecol. 1963;22:773–7.
Gilja BK, Shah VP. Hydrops fetalis due to ABO incompatibility. Clin Pediatr. 1988;27:210–2.
Sherer DM, Abramozicz JS, Ryan RM, Sheils LA, Blumberg N, Woods JR Jr. Severe fetal hydrops resulting from ABO incompatibility. Obstet Gynecol. 1991;78:897–9.
Stiller RJ, Herzlinger R, Siegel S, Whetham JVG. Fetal ascites associated with ABO incompatibility: Case report and review of the literature. Am J Obstet Gyenecol. 1996;175:1371–2.
McDonnell M, Hannam S, Devane SP. Hydrops fetalis due to ABO incompatibility. Arch Dis Child Fetal Neonatal Ed. 1998;78:F220–221.
Tiker F, Gurakan B, Tarcan A, Ozbek N. Fatal course of ABO hemolytic disease associated with hydrops in a twin pregnancy. Turk J Pediatr. 2006;48:73–75.
Drabik-Clary K, Reddy VVB, Benjamin WH, Boctor FN. Severe hemolytic disease of the newborn in a group B African-American infant delivered by a group O mother. Ann Clin Lab Sci. 2006;36:205–7.
Myle AK, Al-Khattabi GH. Hemolytic disease of the newborn; a review of current trends and prospects. Pediatr Health Med Ther. 2021;12:491–8.
Jackson ME, Baker JM. Hemolytic disease of the fetus and newborn: historical and current state. Clin Lab Med. 2021;41:133–51.
Sun JB. The prenatal intervention of pregnancy complicated with anti-Kell isoimmunization: a review. J Matern Fetal Neonatal Med. 2021;34:2893–9.
Coombs RRA, Mourant EA. In-vivo isosensitisation of red cells in babies with haemolytic disease. Lancet. 1946;6391:264–6.
Chown B, Lewis M, Best B. Severe haemolytic disease of the newborn due probably to the combined action of anti-A and anti-S. Can Med Assoc J. 1957;77:31–4.
Denomme GA. Kell and Kx blood group systems. Immunohematology. 2015;31:14–9.
Lee S, Russo D, Redman C. Functional and structural aspects of the kell blood group system. Transfus Med Rev. 2000;14:93–103.
Lee S, Wu X, Reid M, Zelinski T, Redman C. Molecular basis of the Kell (K1) phenotype. Blood. 1995;85:912–6.
Malandrini A, Fabrizi GM, Truschi F, Di Pietro G, Moschini F, Bartalucci P, et al. Atypical McLeod syndrome manifested as X-linked chorea-acanthocytosis, neuromyopathy, and dilated cardiomyopathy: report of a family. J Neurol Sci. 1994;124:89–94.
Vaughan JI, Manning M, Warwick RM, Letsky EA, Murray NA, Roberts IA. Inhibition of erythroid progenitor cells by anti-Kell antibodies in fetal alloimmune anemia. N Engl J Med. 1998;338:798–803.
Wagner T, Lanzer G, Geissler K. Kell expression on myeloid progenitor cells. Leuk Lymphoma. 2002;43:479–85.
Wagner T, Bernaschek G, Geissler K. Inhibition of megakaryopoiesis by Kell-related antibodies. N Engl J Med. 2000;343:72.
Slootweg YM, Lindenburg IT, Koelewijn JM, Van Kamp IL, Oepkes D, De Haas M. Predicting anti-Kell-mediated hemolytic disease of the fetus and newborn: diagnostic accuracy of laboratory management. Am J Obstet Gynecol. 2018;219:393.e1–393.e8.
Rieneck K, Clausen FB, Bergholt T, Nørgaard LN, Dziegiel MH. Non-invasive fetal K status prediction: 7 years of experience. Transfus Med Hemother. 2022;49:240–9.
Ning S, Morin PA, Elahie A, Li N, Liu Y, Barty R, et al. NM. KEL1 negative red cell transfusions for females of current or future child-bearing potential: A clinical impact and feasibility study. Transfusion. 2023;63:59–68.
Luken JS, Folman CC, Lukens MV, Meekers JH, Ligthart PC, Schonewille H, et al. Reduction of anti-K-mediated hemolytic disease of newborns after the introduction of a matched transfusion policy: A nation-wide policy change evaluation study in the Netherlands. Transfusion. 2021;61:713–21.
Cutbush M, Mollison PL, Parkin DM. A new human blood group. Nature. 1950;165:188–9.
Meny GM. The Duffy blood group system: a review. Immunohematology. 2010;26:51–6.
Goodrick MJ, Hadley AG, Poole G. Haemolytic disease of the fetus and newborn due to anti-Fy(a) and the potential clinical value of Duffy genotyping in pregnancies at risk. Transfus Med. 1997;7:301–4.
Tournamille C, Colin Y, Cartron JP, Le Van Kim C. Disruption of a GATA motif in the Duffy gene promoter abolishes erythroid gene expression in Duffy-negative individuals. Nat Genet. 1995;10:224–8.
Atallah-Yunes SA, Ready A, Newburger PE. Benign ethnic neutropenia. Blood Rev. 2019;37:100586.
Gay K, Dulay K, Ravindranath Y, Savaşan S Duffy-null phenotype-associated neutropenia is the most common etiology for leukopenia/neutropenia referrals to a tertiary children’s hospital. J Pediatr. 2023 Jul 5:113608. https://doi.org/10.1016/j.jpeds.2023.113608. Epub ahead of print.
Reid ME. MNS blood group system: a review. Immunohematology. 2009;25:95–101.
Hassan SN, Thirumulu Ponnuraj K, Mohamad S, Hassan R, Wan Ab Rahman WS. Molecular detection of glycophorins A and B variant phenotypes and their clinical relevance. Transfus Med Rev. 2019;33:118–24.
Yasuda H, Ohto H, Nollet KE, Kawabata K, Saito S, Yagi Y, et al. Hemolytic disease of the fetus and newborn with late-onset anemia due to anti-M: a case report and review of the Japanese literature. Transfus Med Rev. 2014;28:1–6.
Andersen LH, Jacob EK, McThenia SS, Tauscher CD, Patterson ER, Oliveira JL, et al. Hemolytic disease and reticulocytopenia of the newborn attributable to maternal immunoglobulin G anti-M reacting optimally at cold temperatures. Transfusion. 2021;61:974–8.
Ishida A, Ohto H, Yasuda H, Negishi Y, Tsuiki H, Arakawa T, et al. Anti-M antibody induced prolonged anemia following hemolytic disease of the newborn due to erythropoietic suppression in 2 siblings. J Pediatr Hematol Oncol. 2015;37:e375–7.
He Y, Gao W, Li Y, Xu C, Wang Q. A single-center, retrospective analysis of 17 cases of hemolytic disease of the fetus and newborn caused by anti-M antibodies. Transfusion. 2023;63:494–506.
Allen FH, Diamond LK, Niedziela B. A new blood-group antigen. Nature. 1951;167:482.
Lawicki S, Covin RB, Powers AA. The Kidd (JK) blood group system. Transfus Med Rev. 2017;31:165–72.
Hamilton JR. Kidd blood group system: a review. Immunohematology. 2015;31:29–35.
Kim WD, Lee YH. A fatal case of severe hemolytic disease of the newborn associated with anti-Jk(b). J Korean Med Sci. 2006;21:151–4.
Velasco Rodríguez D, Pérez-Segura G, Jiménez-Ubieto A, Rodriguez MA, Montejano L. Hemolytic disease of the newborn due to anti-Jkb: case report and review of the literature. Indian J Hematol Blood Transfus. 2014;30:135–8.
Matson GA, Swanson J, Tobin JD. Severe haemolytic disease of the newborn caused by anti-Jka. VoxSanguinis. 1959;4:144–7.
Mittal K, Sood T, Bansal N, Bedi RK, Kaur P, Kaur G. Clinical significance of rare maternal anti Jka antibody. Indian J Hematol Blood Transfus. 2016;32:497–9.
Marchese M. Postpartum acute hemolytic transfusion reactions associated with anti-Lea in two pregnancies complicated by preeclampsia. Immunohematology. 2017;33:114–8.
ACOG Practice Bulletin No. 192: Management of Alloimmunization During Pregnancy. Obstet Gynecol. 2018; 131: e82-e90.
Stenfelt L, Hellberg Å, Westman JS, Olsson ML. The P1PK blood group system: revisited and resolved. Immunohematology. 2020;36:99–103.
Figueroa D. The Diego blood group system: a review. Immunohematology. 2013;29:73–81.
Ting JY, Ma ES, Wong KY. A case of severe haemolytic disease of the newborn due to anti-Di(a) antibody. Hong Kong Med J. 2004;10:347–9.
Hansen TWR, Maisels MJ, Ebbesen F, Vreman HJ, Stevenson DK, Wong RJ, et al. Sixty years of phototherapy for neonatal jaundice - from serendipitous observation to standardized treatment and rescue for millions. J Perinatol. 2020;40:180–93.
Kemper AR, Newman TB, Slaughter JL, Maisels MJ, Watchko JF, Downs SM, et al. Clinical practice guideline revision: management of hyperbilirubinemia in the newborn infant 35 or more weeks of gestation. Pediatrics. 2022;150:e2022058859.
American Academy of Pediatrics Subcommittee on Hyperbilirubinemia. Management of hyperbilirubinemia in the newborn infant 35 or more weeks of gestation. Pediatrics. 2004;114:297–316.
ACOG Practice Bulletin No. 181: Prevention of RhD Alloimmunization. Obstet Gynecol. 2017; 130: e57-e70.
Elsaie AL, Taleb M, Nicosia A, Zangaladze A, Pease ME, Newton K, et al. Comparison of end-tidal carbon monoxide measurements with direct antiglobulin tests in the management of neonatal hyperbilirubinemia. J Perinatol. 2020;40:1513–7.
Vats K, Watchko JF. Coordinating care across the perinatal continuum in hemolytic disease of the fetus and newborn: The timely handoff of a positive maternal anti-erythrocyte antibody screen. J Pediatr. 2019;214:212–6.
Serra de Almeida N, Pinho C, Faim D, Henriques R. Haemolytic disease of the fetus and newborn: do not miss a positive maternal antierythrocyte antibody screen. BMJ Case Rep. 2021;14:e242731.
Author information
Authors and Affiliations
Contributions
RDC, conception, writing first draft, revising manuscript, final approval of the manuscript. TMB, conception, revising manuscript, final approval of the manuscript. SJI, conception, revising manuscript, final approval of the manuscript. DSD-Z, conception, revising manuscript, final approval of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Christensen, R.D., Bahr, T.M., Ilstrup, S.J. et al. Alloimmune hemolytic disease of the fetus and newborn: genetics, structure, and function of the commonly involved erythrocyte antigens. J Perinatol 43, 1459–1467 (2023). https://doi.org/10.1038/s41372-023-01785-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41372-023-01785-3
This article is cited by
-
Erythrokinetic mechanism(s) causing the “late anemia” of hemolytic disease of the fetus and newborn
Journal of Perinatology (2024)