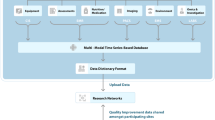
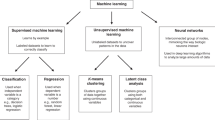
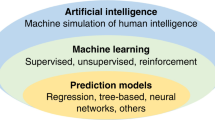
Abstract
Artificial intelligence (AI) has the potential to revolutionize the neonatal intensive care unit (NICU) care by leveraging the large-scale, high-dimensional data that are generated by NICU patients. There is an emerging recognition that the confluence of technological progress, commercialization pathways, and rich data sets provides a unique opportunity for AI to make a lasting impact on the NICU. In this perspective article, we discuss four broad categories of AI applications in the NICU: imaging interpretation, prediction modeling of electronic health record data, integration of real-time monitoring data, and documentation and billing. By enhancing decision-making, streamlining processes, and improving patient outcomes, AI holds the potential to transform the quality of care for vulnerable newborns, making the excitement surrounding AI advancements well-founded and the potential for significant positive change stronger than ever before.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Russell S, Norvig P. A modern, agent-oriented approach to introductory artificial intelligence. SIGART Newsl. 1995;6:24–6.
Finlayson SG, Beam AL, van Smeden M. Machine Learning and Statistics in Clinical Research Articles-Moving Past the False Dichotomy. JAMA Pediatr. 2023. https://doi.org/10.1001/jamapediatrics.2023.0034.
Mccarthy J. What is artificial intelligence? https://cse.unl.edu/~choueiry/S09-476-876/Documents/whatisai.pdf (accessed 2 Feb2023).
Rosenblatt F. The perceptron: a probabilistic model for information storage and organization in the brain. Psychol Rev. 1958;65:386.
Lewis-Kraus G. The great AI awakening. N Y Mag. 2016;14:2016.
Times, T. N. Y. New navy device learns by doing. Psychologist Shows Embryo of Computer Designed to Read and Grow Wiser 1958;74.
Mukherjee, S. AI versus MD: What happens when diagnosis is automated? The New Yorker. 2017;3.
Beam AL, Kohane IS. Translating artificial intelligence into clinical care. JAMA. 2016;316:2368–9.
Yu K-H, Beam AL, Kohane IS. Artificial intelligence in healthcare. Nat Biomed Eng. 2018;2:719–31.
Krizhevsky A, Sutskever I, Hinton GE. ImageNet Classification with Deep Convolutional Neural Networks. In: NIPS. 2012. p. 4.
He K, Zhang X, Ren S, Sun J. Deep residual learning for image recognition. arXiv preprint arXiv:151203385 2015. http://arxiv.org/abs/1512.03385.
Vaswani A, Shazeer N, Parmar N, Uszkoreit J, Jones L, Gomez AN, et al. Attention is all you need. In Proceedings of the 31st International Conference on Neural Information Processing Systems (NIPS'17). Red Hook, NY, USA: Curran Associates Inc.; 2017. 6000–10.
Devlin J, Chang M-W, Lee K, Toutanova K. BERT: Pre-training of Deep Bidirectional Transformers for Language Understanding. arXiv [cs.CL]. 2018. http://arxiv.org/abs/1810.04805.
Schmaltz A, Beam AL. Sharpening the resolution on data matters: a brief roadmap for understanding deep learning for medical data. Spine J. 2021;21:1606–9.
Hinton G, Deng L, Yu D, Dahl GE, Mohamed A-R, Jaitly N, et al. Deep Neural Networks for Acoustic Modeling in Speech Recognition: The Shared Views of Four Research Groups. IEEE Signal Proc Mag. 2012;29:82–97.
Graves A, Mohamed A-R, Hinton G. Speech recognition with deep recurrent neural networks. In: 2013 IEEE International Conference on Acoustics, Speech and Signal Processing. 2013. p. 6645–9.
van den Oord A, Dieleman S, Zen H, Simonyan K, Vinyals O, Graves A, et al. WaveNet: A Generative Model for Raw Audio. arXiv [cs.SD]. 2016. http://arxiv.org/abs/1609.03499.
Lecun Y, Bottou L, Bengio Y, Haffner P. Gradient-based learning applied to document recognition. Proc IEEE. 1998;86:2278–324.
Ciregan D, Meier U, Schmidhuber J. Multi-column deep neural networks for image classification. In: 2012 IEEE Conference on Computer Vision and Pattern Recognition. 2012. p. 3642–9.
LeCun Y, Bengio Y, Hinton G. Deep learning. Nature. 2015;521:436–44.
Schmidhuber J. Deep learning in neural networks: an overview. Neural Netw. 2015;61:85–117.
Redmon J, Divvala S, Girshick R, Farhadi A. You only look once: Unified, real-time object detection. In: 2016 IEEE Conference on Computer Vision and Pattern Recognition (CVPR). IEEE, 2016. p. 779–88.
Brown TB, Mann B, Ryder N, Subbiah M, Kaplan J, Dhariwal P, et al. Language Models are Few-Shot Learners. arXiv [cs.CL]. 2020. http://arxiv.org/abs/2005.14165.
Jumper J, Evans R, Pritzel A, Green T, Figurnov M, Ronneberger O, et al. Highly accurate protein structure prediction with AlphaFold. Nature. 2021;596:583–9.
Ingraham J, Baranov M, Costello Z, Frappier V, Ismail A, Tie S, et al. Illuminating protein space with a programmable generative model. bioRxiv. 2022; 2022.12.01.518682.
Gilmer J, Schoenholz SS, Riley PF, Vinyals O, Dahl GE. Neural Message Passing for Quantum Chemistry. In: Precup D, Teh YW, editors. Proceedings of the 34th International Conference on Machine Learning. PMLR, 06–11 2017. p. 1263–72.
Silver D, Huang A, Maddison CJ, Guez A, Sifre L, van den Driessche G, et al. Mastering the game of Go with deep neural networks and tree search. Nature. 2016;529:484–9.
Beam AL, Drazen JM, Kohane IS, Leong T-Y, Manrai AK, Rubin EJ. Artificial Intelligence in Medicine. N Engl J Med. 2023;388:1220–1.
Beam AL, Kohane IS. Big data and machine learning in health care. J Am Med Assoc. 2018;319:1317–8.
Rajpurkar P, Irvin J, Zhu K, Yang B, Mehta H, Duan T, et al. CheXNet: Radiologist-Level Pneumonia Detection on Chest X-Rays with Deep Learning. arXiv [cs.CV]. 2017. http://arxiv.org/abs/1711.05225.
Gulshan V, Peng L, Coram M, Stumpe MC, Wu D, Narayanaswamy A, et al. Development and Validation of a Deep Learning Algorithm for Detection of Diabetic Retinopathy in Retinal Fundus Photographs. JAMA. 2016;304:649–56.
Kates-Harbeck J, Svyatkovskiy A, Tang W. Predicting disruptive instabilities in controlled fusion plasmas through deep learning. Nature. 2019;568:526–31.
Baldi P, Sadowski P, Whiteson D. Searching for exotic particles in high-energy physics with deep learning. Nat Commun. 2014;5:4308.
AlQuraishi M. AlphaFold at CASP13. Bioinformatics. 2019. https://doi.org/10.1093/bioinformatics/btz422.
Moult J. A decade of CASP: progress, bottlenecks and prognosis in protein structure prediction. Curr Opin Struct Biol. 2005;15:285–9.
Ghorbani A, Ouyang D, Abid A, He B, Chen JH, Harrington RA, et al. Deep learning interpretation of echocardiograms. NPJ Digit Med. 2020;3:10.
Hughes JW, Yuan N, He B, Ouyang J, Ebinger J, Botting P, et al. Deep learning prediction of biomarkers from echocardiogram videos. bioRxiv. 2021. https://doi.org/10.1101/2021.02.03.21251080.
Soenksen LR, Kassis T, Conover ST, Marti-Fuster B, Birkenfeld JS, Tucker-Schwartz J, et al. Using deep learning for dermatologist-level detection of suspicious pigmented skin lesions from wide-field images. Sci Transl Med. 2021;13. https://doi.org/10.1126/scitranslmed.abb3652.
Liu Y, Jain A, Eng C, Way DH, Lee K, Bui P, et al. A deep learning system for differential diagnosis of skin diseases. Nat Med. 2020;26:900–8.
Esteva A, Kuprel B, Novoa RA, Ko J, Swetter SM, Blau HM, et al. Corrigendum: Dermatologist-level classification of skin cancer with deep neural networks. Nature. 2017;546:686.
Abràmoff MD, Lavin PT, Birch M, Shah N, Folk JC. Pivotal trial of an autonomous AI-based diagnostic system for detection of diabetic retinopathy in primary care offices. NPJ Digit Med. 2018;1:39.
Abràmoff MD, Lou Y, Erginay A, Clarida W, Amelon R, Folk JC, et al. Improved Automated Detection of Diabetic Retinopathy on a Publicly Available Dataset Through Integration of Deep Learning. Invest Ophthalmol Vis Sci. 2016;57:5200–6.
Campanella G, Hanna MG, Geneslaw L, Miraflor A, Werneck Krauss Silva V, Busam KJ, et al. Clinical-grade computational pathology using weakly supervised deep learning on whole slide images. Nat Med. 2019;25:1301–9.
Janowczyk A, Madabhushi A. Deep learning for digital pathology image analysis: A comprehensive tutorial with selected use cases. J Pathol Inf. 2016;7:29.
McBee MP, Awan OA, Colucci AT, Ghobadi CW, Kadom N, Kansagra AP, et al. Deep Learning in Radiology. Acad Radio. 2018;25:1472–80.
Palepu A, Beam AL. TIER: Text-Image Entropy Regularization for CLIP-style models. arXiv preprint arXiv:2212 06710 2022.
Wu E, Wu K, Daneshjou R, Ouyang D, Ho DE, Zou J. How medical AI devices are evaluated: limitations and recommendations from an analysis of FDA approvals. Nat Med. 2021;27:582–4.
Cruz Rivera S, Liu X, Chan A-W, Denniston AK, Calvert MJ, SPIRIT-AI and CONSORT-AI Working Group. Guidelines for clinical trial protocols for interventions involving artificial intelligence: the SPIRIT-AI extension. Lancet Digit Health. 2020;2:e549–e560.
Liu X, Rivera SC, Moher D, Calvert MJ, Denniston AK, Ashrafian H, et al. Reporting guidelines for clinical trial reports for interventions involving artificial intelligence: the CONSORT-AI extension. The Lancet Digital Health, 2020;2:pp.e537-e548.
Rasmy L, Xiang Y, Xie Z, Tao C, Zhi D. Med-BERT: pretrained contextualized embeddings on large-scale structured electronic health records for disease prediction. NPJ Digit Med. 2021;4:86.
Rajkomar A, Oren E, Chen K, Dai AM, Hajaj N, Hardt M, et al. Scalable and accurate deep learning with electronic health records. NPJ Digit Med. 2018;1:18.
Davoudi A, Malhotra KR, Shickel B, Siegel S, Williams S, Ruppert M, et al. Intelligent ICU for Autonomous Patient Monitoring Using Pervasive Sensing and Deep Learning. Sci Rep. 2019;9:8020.
Mathews SM, Kambhamettu C, Barner KE. A novel application of deep learning for single-lead ECG classification. Comput Biol Med. 2018;99:53–62.
Nestor B, Hunter J, Kainkaryam R, Drysdale E, Inglis JB, Shapiro A, et al. Machine learning COVID-19 detection from wearables. Lancet Digit Health. 2023;5:e182–e184.
Schulman J, Zoph B, Kim C, Hilton J, Menick J, Weng J, et al. ChatGPT: Optimizing language models for dialogue. https://openai.com/blog/chatgpt (accessed 11 Jul2023).
Lee P, Bubeck S, Petro J. Benefits, limits, and risks of GPT-4 as an AI chatbot for medicine. N Engl J Med. 2023;388:1233–9.
Pichai S. An important next step on our AI journey. Google. 2023. https://blog.google/intl/en-africa/products/explore-get-answers/an-important-next-step-on-our-ai-journey/ (accessed 11 Jul2023).
Levine DM, Tuwani R, Kompa B, Varma A, Finlayson SG, Mehrotra A, et al. The Diagnostic and Triage Accuracy of the GPT-3 Artificial Intelligence Model. medRxiv 2023; 2023–2001.
Kung TH, Cheatham M, Medinilla A, Sillos C, De Leon L, Elepano C, et al. Performance of ChatGPT on USMLE: Potential for AI-Assisted Medical Education Using Large Language Models. medRxiv. 2022; 2022–34.
Singhal K, Azizi S, Tu T, Sara Mahdavi S, Wei J, Chung HW, et al. Large Language Models Encode Clinical Knowledge. arXiv [cs.CL]. 2022. http://arxiv.org/abs/2212.13138.
Chen MM, Golding LP, Nicola GN. Who Will Pay for AI? Radio Artif Intell. 2021;3:e210030.
Abràmoff MD, Roehrenbeck C, Trujillo S, Goldstein J, Graves AS, Repka MX, et al. A reimbursement framework for artificial intelligence in healthcare. NPJ Digit Med. 2022;5:72.
Redd TK, Campbell JP, Brown JM, Kim SJ, Ostmo S, Chan RVP, et al. Evaluation of a deep learning image assessment system for detecting severe retinopathy of prematurity. Br J Ophthalmol. 2018. https://doi.org/10.1136/bjophthalmol-2018-313156.
Taylor S, Brown JM, Gupta K, Campbell JP, Ostmo S, Chan RVP, et al. Monitoring Disease Progression With a Quantitative Severity Scale for Retinopathy of Prematurity Using Deep Learning. JAMA Ophthalmol. 2019;137:1022–8.
Tan Z, Simkin S, Lai C, Dai S. Deep Learning Algorithm for Automated Diagnosis of Retinopathy of Prematurity Plus Disease. Transl Vis Sci Technol. 2019;8:23.
Maeda R, Fujita D, Tanaka K, Ozawa J, Haga M, Miyahara H, et al. Predicting the severity of Neonatal Chronic Lung Disease from chest X-ray images using deep learning. In: 2022 IEEE International Conference on Systems, Man, and Cybernetics (SMC). 2022. p. 1543–7.
Poplin R, Varadarajan AV, Blumer K, Liu Y, McConnell MV, Corrado GS, et al. Prediction of cardiovascular risk factors from retinal fundus photographs via deep learning. Nat Biomed Eng. 2018;2:158–64.
Ramgopal S, Horvat CM, Yanamala N, Alpern ER. Machine Learning To Predict Serious Bacterial Infections in Young Febrile Infants. Pediatrics 2020; 146. https://doi.org/10.1542/peds.2019-4096.
Hsu J-F, Chang Y-F, Cheng H-J, Yang C, Lin C-Y, Chu S-M, et al. Machine Learning Approaches to Predict In-Hospital Mortality among Neonates with Clinically Suspected Sepsis in the Neonatal Intensive Care Unit. J Pers Med. 2021;11. https://doi.org/10.3390/jpm11080695.
Zeigler AC, Ainsworth JE, Fairchild KD, Wynn JL, Sullivan BA. Sepsis and mortality prediction in very low birth weight infants: Analysis of HeRO and nSOFA. Am J Perinatol. 2023;40:407–14.
Irles C, González-Pérez G, Carrera Muiños S, Michel Macias C, Sánchez Gómez C, Martínez-Zepeda A, et al. Estimation of Neonatal Intestinal Perforation Associated with Necrotizing Enterocolitis by Machine Learning Reveals New Key Factors. Int J Environ Res Public Health. 2018;15. https://doi.org/10.3390/ijerph15112509.
Beam KS, Lee M, Hirst K, Beam A, Parad RB. Specificity of International Classification of Diseases codes for bronchopulmonary dysplasia: an investigation using electronic health record data and a large insurance database. J Perinatol. 2021;41:764–71.
McAdams RM, Kaur R, Sun Y, Bindra H, Cho SJ, Singh H. Predicting clinical outcomes using artificial intelligence and machine learning in neonatal intensive care units: a systematic review. J Perinatol. 2022;42:1561–75.
Fairchild KD, O’Shea TM. Heart rate characteristics: physiomarkers for detection of late-onset neonatal sepsis. Clin Perinatol. 2010;37:581–98.
Fairchild KD, Lake DE, Kattwinkel J, Moorman JR, Bateman DA, Grieve PG, et al. Vital signs and their cross-correlation in sepsis and NEC: a study of 1,065 very-low-birth-weight infants in two NICUs. Pediatr Res. 2017;81:315–21.
Fairchild, KD, Aschner, JL. HeRO monitoring to reduce mortality in NICU patients. Research and Reports in Neonatology; 2012. pp. 65–76.
Boyd E. Introducing GPT-4 in Azure OpenAI Service. https://azure.microsoft.com/en-us/blog/introducing-gpt4-in-azure-openai-service/ (accessed 1 Apr2023).
Zhang H, Lu AX, Abdalla M, McDermott M, Ghassemi M. Hurtful words: quantifying biases in clinical contextual word embeddings. In: Proceedings of the ACM Conference on Health, Inference, and Learning. New York, NY, USA: Association for Computing Machinery; 2020. p. 110–20.
Chen IY, Pierson E, Rose S, Joshi S, Ferryman K, Ghassemi M. Ethical machine learning in healthcare. Annu Rev Biomed Data Sci. 2021;4:123–44.
Obermeyer Z, Powers B, Vogeli C, Mullainathan S. Dissecting racial bias in an algorithm used to manage the health of populations. Science. 2019;366:447–53.
Finlayson SG, Subbaswamy A, Singh K, Bowers J, Kupke A, Zittrain J, et al. The clinician and dataset shift in artificial intelligence. N. Engl J Med. 2021;385:283–6.
Kompa B, Snoek J, Beam A. Empirical Frequentist Coverage of Deep Learning Uncertainty Quantification Procedures. Entropy. 2021. https://www.mdpi.com/1099-4300/23/12/1608.
Wong A, Otles E, Donnelly JP, Krumm A, McCullough J, DeTroyer-Cooley O, et al. External validation of a widely implemented proprietary sepsis prediction model in hospitalized patients. JAMA Intern Med. 2021;181:1065–70.
Ghassemi M, Oakden-Rayner L, Beam AL. The false hope of current approaches to explainable artificial intelligence in health care. Lancet Digit Health. 2021;3:e745–e750.
Mündler N, He J, Jenko S, Vechev M. Self-contradictory hallucinations of large language models: Evaluation, detection and mitigation. arXiv [cs.CL]. 2023. http://arxiv.org/abs/2305.15852.
OpenAI. GPT-4 Technical Report. arXiv [cs.CL]. 2023. http://arxiv.org/abs/2303.08774.
Acknowledgements
The authors wish to disclose that ChatGPT based on GPT-4 was used to edit and revise some passages in this paper. Specifically, ChatGPT was used as a writing aid to assist in refining human-generated text to enhance the overall clarity of the writing. However, the intellectual contributions, ideas, and conclusions presented herein are exclusively the product of the authors.
Author information
Authors and Affiliations
Contributions
KB and ALB conceived of initial manuscript concept. KB, PS, PL, and ALB wrote, edited, and approved of the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Beam, K., Sharma, P., Levy, P. et al. Artificial intelligence in the neonatal intensive care unit: the time is now. J Perinatol 44, 131–135 (2024). https://doi.org/10.1038/s41372-023-01719-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41372-023-01719-z