Abstract
Objectives
Determine if chronologic age and/or chorioamnionitis exposure alter normal serum cytokine and chemokine levels in uninfected preterm neonates during their initial NICU stay.
Study design
A 7-plex immunoassay measured levels of serum IL-1β, IL-6, IL-8, IL-10, TNF-α, CCL2, and CCL3 longitudinally from chorioamnionitis-exposed and unexposed preterm neonates under 33 weeks’ gestation.
Results
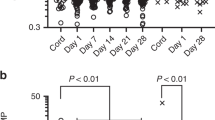
Chorioamnionitis-exposed and unexposed preterm neonates demonstrated differences in the trends of IL-1β, IL-6, IL-8, IL-10, TNF-α, and CCL2 over the first month of life. The unexposed neonates demonstrated elevated levels of these inflammatory markers in the first two weeks of life with a decrease by the third week of life, while the chorioamnionitis-exposed neonates demonstrated differences over time without a predictable pattern. Chorioamnionitis-exposed and unexposed neonates demonstrated altered IL-10 and TNF-α trajectories over the first twelve weeks of life.
Conclusion
Chorioamnionitis induces a state of immune dysregulation in preterm neonates that persists beyond the immediate neonatal period.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Blencowe H, Cousens S, Oestergaard MZ, Chou D, Moller AB, Narwal R, et al. National, regional, and worldwide estimates of preterm birth rates in the year 2010 with time trends since 1990 for selected countries: a systematic analysis and implications. Lancet. 2012;379:2162–72.
Simonsen KA, Anderson-Berry AL, Delair SF, Davies HD. Early-onset neonatal sepsis. Clin Microbiol Rev. 2014;27:21–47.
Matoba N, Yu Y, Mestan K, Pearson C, Ortiz K, Porta N, et al. Differential patterns of 27 cord blood immune biomarkers across gestational age. Pediatrics. 2009;123:1320–8.
Lusyati S, Hulzebos CV, Zandvoort J, Sauer PJ. Levels of 25 cytokines in the first seven days of life in newborn infants. BMC Res Notes. 2013;6:547.
Salio M, Speak AO, Shepherd D, Polzella P, Illarionov PA, Veerapen N, et al. Modulation of human natural killer T cell ligands on TLR-mediated antigen-presenting cell activation. Proc Natl Acad Sci USA. 2007;104:20490–5.
Peng CC, Chang JH, Lin HY, Cheng PJ, Su BH. Intrauterine inflammation, infection, or both (Triple I): A new concept for chorioamnionitis. Pediatr Neonatol. 2018;59:231–7.
Romero R, Chaemsaithong P, Docheva N, Korzeniewski SJ, Tarca AL, Bhatti G, et al. Clinical chorioamnionitis at term V: umbilical cord plasma cytokine profile in the context of a systemic maternal inflammatory response. J Perinat Med. 2016;44:53–76.
de Jong E, Hancock DG, Wells C, Richmond P, Simmer K, Burgner D, et al. Exposure to chorioamnionitis alters the monocyte transcriptional response to the neonatal pathogen Staphylococcus epidermidis. Immunol Cell Biol. 2018;96:792–804.
Bermick J, Gallagher K, denDekker A, Kunkel S, Lukacs N, Schaller M. Chorioamnionitis exposure remodels the unique histone modification landscape of neonatal monocytes and alters the expression of immune pathway genes. FEBS J. 2019;286:82–109.
Schrag SJ, Hadler JL, Arnold KE, Martell-Cleary P, Reingold A, Schuchat A. Risk factors for invasive, early-onset Escherichia coli infections in the era of widespread intrapartum antibiotic use. Pediatrics. 2006;118:570–6.
Garcia-Munoz Rodrigo F, Galan Henriquez G, Figueras Aloy J, Garcia-Alix, Perez A. Outcomes of very-low-birth-weight infants exposed to maternal clinical chorioamnionitis: a multicentre study. Neonatology. 2014;106:229–34.
Redline RW, Faye-Petersen O, Heller D, Qureshi F, Savell V, Vogler C, et al. Amniotic infection syndrome: nosology and reproducibility of placental reaction patterns. Pediatr Dev Pathol. 2003;6:435–48.
Khong TY, Mooney EE, Ariel I, Balmus NC, Boyd TK, Brundler MA, et al. Sampling and definitions of placental lesions: amsterdam placental workshop group consensus statement. Arch Pathol Lab Med. 2016;140:698–713.
Khaertynov KS, Boichuk SV, Khaiboullina SF, Anokhin VA, Andreeva AA, Lombardi VC, et al. Comparative assessment of cytokine pattern in early and late onset of neonatal sepsis. J Immunol Res. 2017;2017:8601063.
Leviton A, O’Shea TM, Bednarek FJ, Allred EN, Fichorova RN, Dammann O. Systemic responses of preterm newborns with presumed or documented bacteraemia. Acta Paediatr. 2012;101:355–9.
Harris MC, Costarino AT Jr., Sullivan JS, Dulkerian S, McCawley L, Corcoran L, et al. Cytokine elevations in critically ill infants with sepsis and necrotizing enterocolitis. J Pediatr. 1994;124:105–11.
Edelson MB, Bagwell CE, Rozycki HJ. Circulating pro- and counterinflammatory cytokine levels and severity in necrotizing enterocolitis. Pediatrics 1999;103:766–71.
Robison HM, Bailey RC. A guide to quantitative biomarker assay development using whispering gallery mode biosensors. Curr Protoc Chem Biol. 2017;9:158–73.
Robison HM, Escalante P, Valera E, Erskine CL, Auvil L, Sasieta HC, et al. Precision immunoprofiling to reveal diagnostic signatures for latent tuberculosis infection and reactivation risk stratification. Integr Biol (Camb). 2019;11:16–25.
Mudumba S, de Alba S, Romero R, Cherwien C, Wu A, Wang J, et al. Photonic ring resonance is a versatile platform for performing multiplex immunoassays in real time. J Immunol Methods. 2017;448:34–43.
Ofman G, Vasco N, Cantey JB. Risk of early-onset sepsis following preterm, prolonged rupture of membranes with or without chorioamnionitis. Am J Perinatol. 2016;33:339–42.
Strunk T, Doherty D, Jacques A, Simmer K, Richmond P, Kohan R, et al. Histologic chorioamnionitis is associated with reduced risk of late-onset sepsis in preterm infants. Pediatrics 2012;129:e134–41.
Puri K, Taft DH, Ambalavanan N, Schibler KR, Morrow AL, Kallapur SG. Association of chorioamnionitis with aberrant neonatal gut colonization and adverse clinical outcomes. PLoS One. 2016;11:e0162734.
Villamor-Martinez E, Lubach GA, Rahim OM, Degraeuwe P, Zimmermann LJ, Kramer BW, et al. Association of histological and clinical chorioamnionitis with neonatal sepsis among preterm infants: a systematic review, meta-analysis, and meta-regression. Front Immunol. 2020;11:972.
Canto E, Rodriguez-Sanchez JL, Vidal S. Distinctive response of naive lymphocytes from cord blood to primary activation via TCR. J Leukoc Biol. 2003;74:998–1007.
Marodi L. Down-regulation of Th1 responses in human neonates. Clin Exp Immunol. 2002;128:1–2.
Iroh Tam PY, Bendel CM. Diagnostics for neonatal sepsis: current approaches and future directions. Pediatr Res. 2017;82:574–83.
Kinjo T, Ohga S, Ochiai M, Honjo S, Tanaka T, Takahata Y, et al. Serum chemokine levels and developmental outcome in preterm infants. Early Hum Dev. 2011;87:439–43.
Franz AR, Steinbach G, Kron M, Pohlandt F. Interleukin-8: a valuable tool to restrict antibiotic therapy in newborn infants. Acta Paediatr. 2001;90:1025–32.
de Bont ES, Martens A, van Raan J, Samson G, Fetter WP, Okken A, et al. Tumor necrosis factor-alpha, interleukin-1 beta, and interleukin-6 plasma levels in neonatal sepsis. Pediatr Res. 1993;33:380–3.
Dulay AT, Buhimschi IA, Zhao G, Bahtiyar MO, Thung SF, Cackovic M, et al. Compartmentalization of acute phase reactants interleukin-6, C-reactive protein and procalcitonin as biomarkers of intra-amniotic infection and chorioamnionitis. Cytokine 2015;76:236–43.
Ye Q, Du LZ, Shao WX, Shang SQ. Utility of cytokines to predict neonatal sepsis. Pediatr Res. 2017;81:616–21.
Sarandakou A, Giannaki G, Malamitsi-Puchner A, Rizos D, Hourdaki E, Protonotariou E, et al. Inflammatory cytokines in newborn infants. Mediators Inflamm. 1998;7:309–12.
Protonotariou E, Malamitsi-Puchner A, Giannaki G, Rizos D, Phocas I, Sarandakou A. Patterns of inflammatory cytokine serum concentrations during the perinatal period. Early Hum Dev. 1999;56:31–8.
Reinhart K, Bauer M, Riedemann NC, Hartog CS. New approaches to sepsis: molecular diagnostics and biomarkers. Clin Microbiol Rev. 2012;25:609–34.
Kocabas E, Sarikcioglu A, Aksaray N, Seydaoglu G, Seyhun Y, Yaman A. Role of procalcitonin, C-reactive protein, interleukin-6, interleukin-8 and tumor necrosis factor-alpha in the diagnosis of neonatal sepsis. Turk J Pediatr. 2007;49:7–20.
Leviton A, O’Shea TM, Bednarek FJ, Allred EN, Fichorova RN, Dammann O, et al. Systemic responses of preterm newborns with presumed or documented bacteraemia. Acta Paediatr. 2012;101:355–9.
Maheshwari A, Schelonka RL, Dimmitt RA, Carlo WA, Munoz-Hernandez B, Das A, et al. Cytokines associated with necrotizing enterocolitis in extremely-low-birth-weight infants. Pediatr Res. 2014;76:100–8.
Sahni M, Yeboah B, Das P, Shah D, Ponnalagu D, Singh H, et al. Novel biomarkers of bronchopulmonary dysplasia and bronchopulmonary dysplasia-associated pulmonary hypertension. J Perinatol. 2020;40:1634–43.
Kumar R, Yu Y, Story RE, Pongracic JA, Gupta R, Pearson C, et al. Prematurity, chorioamnionitis, and the development of recurrent wheezing: a prospective birth cohort study. J Allergy Clin Immunol. 2008;121:878–84 e6.
Schmatz M, Srinivasan L, Grundmeier RW, Elci OU, Weiss SL, Masino AJ, et al. Surviving sepsis in a referral neonatal intensive care unit: association between time to antibiotic administration and in-hospital outcomes. J Pediatr. 2020;217:59–65 e1.
Venkatesh M, Flores A, Luna RA, Versalovic J. Molecular microbiological methods in the diagnosis of neonatal sepsis. Expert Rev Anti Infect Ther. 2010;8:1037–48.
Sharma D, Farahbakhsh N, Shastri S, Sharma P. Biomarkers for diagnosis of neonatal sepsis: a literature review. J Matern Fetal Neonatal Med. 2018;31:1646–59.
Sharma AA, Jen R, Kan B, Sharma A, Marchant E, Tang A, et al. Impaired NLRP3 inflammasome activity during fetal development regulates IL-1beta production in human monocytes. Eur J Immunol. 2015;45:238–49.
Strunk T, Prosser A, Levy O, Philbin V, Simmer K, Doherty D, et al. Responsiveness of human monocytes to the commensal bacterium Staphylococcus epidermidis develops late in gestation. Pediatr Res. 2012;72:10–8.
Jakobsson HE, Abrahamsson TR, Jenmalm MC, Harris K, Quince C, Jernberg C, et al. Decreased gut microbiota diversity, delayed Bacteroidetes colonisation and reduced Th1 responses in infants delivered by caesarean section. Gut. 2014;63:559–66.
Funding
This project was funded through philanthropic funds from the Korneffel family and by the National Institute of Allergy and Infectious Diseases at the National Institutes of Health AI141673.
Author information
Authors and Affiliations
Contributions
GES made substantial contributions to the acquisition, analysis and interpretation of the data and wrote the initial draft of the manuscript. CAC and KLM made substantial contributions to the acquisition, analysis and interpretation of the data and critically revised the manuscript. JMS made substantial contributions to the analysis and interpretation of the data and critically revised the manuscript. LAE made substantial contributions to the design of the work, analysis and interpretation of the data and critically revised the manuscript. RCB and JRB made substantial contributions to the conceptualization and design of the work, acquisition, analysis and interpretation of the data and critically revised the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Stepanovich, G.E., Chapman, C.A., Meserve, K.L. et al. Chorioamnionitis-exposure alters serum cytokine trends in premature neonates. J Perinatol 43, 758–765 (2023). https://doi.org/10.1038/s41372-022-01584-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41372-022-01584-2