Abstract
Objective
To investigate whether NICU discharge summaries documented neonatal AKI and estimate if nephrology consultation mediated this association.
Study design
Secondary analysis of AWAKEN multicenter retrospective cohort. Exposures: AKI severity and diagnostic criteria. Outcome: AKI documentation on NICU discharge summaries using multivariable logistic regression to estimate associations and test for causal mediation.
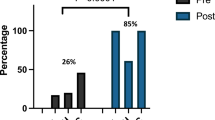
Results
Among 605 neonates with AKI, 13% had documented AKI. Those with documented AKI were more likely to have severe AKI (70.5% vs. 51%, p < 0.001) and SCr-only AKI (76.9% vs. 50.1%, p = 0.04). Nephrology consultation mediated 78.0% (95% CL 46.5–109.4%) of the total effect of AKI severity and 82.8% (95% CL 70.3–95.3%) of the total effect of AKI diagnostic criteria on documentation.
Conclusion
We report a low prevalence of AKI documentation at NICU discharge. AKI severity and SCr-only AKI increased odds of AKI documentation. Nephrology consultation mediated the associations of AKI severity and diagnostic criteria with documentation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
Data availability
De‐identified individual participant data will be made available through the Neonatal Kidney Collaborative through their website www.babykidney.org. The data will be de‐identified and a limited access data set is available through a request form on that page. Data dictionaries, in addition to study protocol, and case report forms will be made available. The data will be made available to researchers who provide a methodologically sound proposal for use in achieving the goals of the approved proposal.
References
Jetton JG, Boohaker LJ, Sethi SK, Wazir S, Rohatgi S, Soranno DE, et al. Incidence and outcomes of neonatal acute kidney injury (AWAKEN): a multicentre, multinational, observational cohort study. Lancet Child Adolesc Health. 2017;1:184–94.
Starr MC, Charlton JR, Guillet R, Reidy K, Tipple TE, Jetton JG, et al. Advances in neonatal acute kidney injury. Pediatrics. United States: © 2021 by the American Academy of Pediatrics.; 2021.
Khwaja A. KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin Pr. 2012;120:c179–84.
Carmody JB, Swanson JR, Rhone ET, Charlton JR. Recognition and reporting of AKI in very low birth weight infants. Clin J Am Soc Nephrol. 2014;9:2036–43.
Vincent K, Murphy HJ, Ross JR, Twombley KE. Acute kidney injury guidelines are associated with improved recognition and follow-up for neonatal patients. Adv Neonatal Care. 2020;20:269–75.
Starr MC, Kula A, Lieberman J, Lam T, Chabra S, Hingorani S. Improving the recognition and reporting of acute kidney injury in the neonatal intensive care unit. J Perinatol. 2020;40:1301–7.
Starr MC, Chaudhry P, Brock A, Vincent K, Twombley K, Bonachea EM, et al. Improving the identification of acute kidney injury in the neonatal ICU: three centers’ experiences. J Perinatol. United States: © 2021. The Author(s), under exclusive licence to Springer Nature America, Inc.; 2021.
Cohen JB, D’Agostino McGowan L, Jensen ET, Rigdon J, South AM. Evaluating sources of bias in observational studies of angiotensin-converting enzyme inhibitor/angiotensin II receptor blocker use during COVID-19: beyond confounding. J Hypertens. 2021;39:795–805.
Jetton JG, Guillet R, Askenazi DJ, Dill L, Jacobs J, Kent AL, et al. Assessment of worldwide acute kidney injury epidemiology in neonates: design of a retrospective cohort study. Front Pediatr. 2016;4:68.
Shrier I, Platt RW. Reducing bias through directed acyclic graphs. BMC Med Res Methodol. 2008;8:70.
Textor J, van der Zander B, Gilthorpe MS, Liskiewicz M, Ellison GT. Robust causal inference using directed acyclic graphs: the R package ‘dagitty’. Int J Epidemiol. 2016;45:1887–94.
Askenazi DJ. AWAKEN-Ing a new frontier in neonatal nephrology. Front Pediatr. 2020;8:21.
Askenazi DJ, Ambalavanan N, Goldstein SL. Acute kidney injury in critically ill newborns: what do we know? What do we need to learn? Pediatr Nephrol. 2009;24:265–74.
Zappitelli M, Ambalavanan N, Askenazi DJ, Moxey-Mims MM, Kimmel PL, Star RA, et al. Developing a neonatal acute kidney injury research definition: a report from the NIDDK neonatal AKI workshop. Pediatr Res. 2017;82:569–73.
Askenazi D, Abitbol C, Boohaker L, Griffin R, Raina R, Dower J, et al. Optimizing the AKI definition during first postnatal week using Assessment of Worldwide Acute Kidney Injury Epidemiology in Neonates (AWAKEN) cohort. Pediatr Res. 2019;85:329–38.
Goldstein SL. Urine output assessment in acute kidney injury: the cheapest and most impactful biomarker. Front Pediatr. 2019;7:565.
Kent AL, Charlton JR, Guillet R, Gist KM, Hanna M, El Samra A, et al. Neonatal acute kidney injury: a survey of neonatologists’ and nephrologists’ perceptions and practice management. Am J Perinatol. 2018;35:1–9.
Harer MW, Charlton JR, Tipple TE, Reidy KJ. Preterm birth and neonatal acute kidney injury: implications on adolescent and adult outcomes. J Perinatol. 2020;40:1286–95. https://doi.org/10.1038/s41372-020-0656-7.
Mammen C, Al Abbas A, Skippen P, Nadel H, Levine D, Collet JP, et al. Long-term risk of CKD in children surviving episodes of acute kidney injury in the intensive care unit: a prospective cohort study. Am J Kidney Dis. 2012;59:523–30.
Selewski DT, Hyatt DM, Bennett KM, Charlton JR. Is acute kidney injury a harbinger for chronic kidney disease? Curr Opin Pediatr. 2018;30:236–40.
VanderWeele TJ. A unification of mediation and interaction: a 4-way decomposition. Epidemiology 2014;25:749–61.
Acknowledgements
The authors would like to thank Samantha Wallace (Department of Pediatrics, Indiana University School of Medicine) for help with technical editing and proofreading of this manuscript.
Funding
Cincinnati Children’s Hospital Center for Acute Care Nephrology provided funding to create and maintain the Assessment of Worldwide Acute Kidney Injury Epidemiology in Neonates (AWAKEN) study Medidata Rave electronic database. The Pediatric and Infant Center for Acute Nephrology (PICAN) provided support for web meetings and for the Neonatal Kidney Collaborative (NKC) steering committee annual meeting at The University of Alabama at Birmingham (UAB), as well as support for 2 of the AWAKEN study investigators at UAB (D.A. and R.G.). PICAN is part of the Department of Pediatrics at UAB and is funded by Children’s of Alabama hospital, UAB Department of Pediatrics, UAB School of Medicine, and UAB Center for Clinical and Translational Sciences (National Institutes of Health grant UL1TR001417). Finally, the AWAKEN study at The University of New Mexico was supported by the Clinical and Translational Science Center at The University of New Mexico (National Institutes of Health grant UL1TR001449) and by The University of Iowa Institute for Clinical and Translational Science (grant U54TR001356). The AWAKEN study investigators at the Canberra Hospital at the Australian National University Medical School were supported by the Canberra Hospital Private Practice Fund, and investigators at University of Virginia Children’s Hospital were supported by a 100 Women Who Care Grant from the 100 Women Charitable Foundation. The funding sources for this study had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Author information
Authors and Affiliations
Consortia
Contributions
JC and MS conceptualized and designed the study, assisted with data analysis and interpretation, drafted the initial manuscript, and reviewed and revised the manuscript. AC and RG performed statistical analysis and reviewed and revised the manuscript. DA and JJ conceptualized and designed the study, provided support and mentorship, and reviewed and revised the manuscript. PC, MH, SM and AS assisted with the design of the study, data interpretation, and reviewed and revised the manuscript for important intellectual content. All authors approve the final manuscript as submitted and agree to be accountable for all aspects of the work.
Corresponding author
Ethics declarations
Competing interests
All authors report no real or perceived conflicts of interest that could affect the study design, collection, analysis, and interpretation of data, the writing of the report, or the decision to submit the manuscript for publication. For full disclosure, we provide the additional list of authors’ other funding not directly related to this study. DA consults for and received research/education grants with Baxter, Medtronic, Nuwellis, Bioporto and Seastar. He has patent pending inventions in the area of neonatal nephrology. AS reports receiving grant funding for studies not related to this work, grants NHLBI K23 HL148394, L40 HL148910, and R01 HL146818. MS is supported in part by the Indiana University School of Medicine Physician Scientist Initiative. No other disclosures were reported.
Ethics approval and consent to participate
The Institutional Review Board (IRB) at the University of Alabama at Birmingham approved this collaborative multicenter study, and each center received approval from their respective IRBs for participation. The study design allowed for a waiver of informed consent or parental permission. This study is registered with ClinicalTrials.org, number NCT02443389.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Chmielewski, J., Chaudhry, P.M., Harer, M.W. et al. Documentation of acute kidney injury at discharge from the neonatal intensive care unit and role of nephrology consultation. J Perinatol 42, 930–936 (2022). https://doi.org/10.1038/s41372-022-01424-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41372-022-01424-3
This article is cited by
-
Utilizing electronic medical records alert to improve documentation of neonatal acute kidney injury
Pediatric Nephrology (2024)