Abstract
Objective
To estimate the incidence of hypophosphatemia in preterm infants according to parenteral nutrition received and to evaluate associated risk factors.
Design
A prospective multicenter cohort study included 111 patients ≤ 1250 g (7 NICUs of the NEOCOSUR Network). Two groups were compared according to the amino-acid supply in the first 48 h: aggressive parenteral group ≥ 3 g/kg/day and standard parenteral group: <2.9 g/kg/day. Hypophosphatemia was defined as serum phosphate < 4 mg/dl. A logistic regression analysis was performed to evaluate associated risk factors.
Results
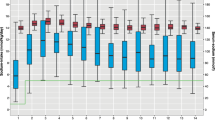
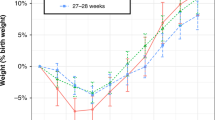
Fifty-eight infants received aggressive parenteral nutrition. The incidence of hypophosphatemia was significantly higher in the aggressive parenteral group (77.5% vs 53.8%, p = 0.009). Hypophosphatemia was independently associated with aggressive parenteral nutrition (aOR 4.16 95% CI 1.54–12.24) and negatively associated with phosphorous intake (aOR 0.92 95% CI 0.87–0.97).
Conclusion
Both high amino-acid intake and low phosphorus supply during the first days after birth were independently associated with hypophosphatemia.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Van Den Akker CH, Vlaardingerbroek H, Van Goudoever JB. Nutritional support for extremely low-birth weight infants: abandoning catabolism in the neonatal intensive care unit. Curr Opin Clin Nutr Metab Care. 2010;13:327–35.
Thureen PJ. Early aggressive nutrition in the neonate. Pediatr Rev. 2007;20:45e–55.
Senterre T, Rigo J. Reduction in postnatal cumulative nutritional deficit and improvement of growth in extremely preterm infants. Acta Paediatr. 2012;101:64–70.
Dinerstein A, Nieto RM, Solana CL, Perez GP, Otheguy LE, Larguia AM. Early and aggressive nutritional strategy (parenteral and enteral) decreases postnatal growth failure in very low birth weight infants. J Perinatol. 2006;26:436–42.
Ehrenkranz RA. Early, aggressive nutritional management for very low birth weight infants: what is the evidence? Semin Perinatol. 2007;31:48–55.
Hay WW Jr. Aggressive nutrition of the preterm infant. Curr Pediatr Rep. 2013;1:1–17.
Senterre T, Abu Zahirah I, Pieltain C, de Halleux V, Rigo J. Electrolyte and mineral homeostasis after optimizing early macronutrient intakes in VLBW infants on parenteral nutrition. J Pediatr Gastroenterol Nutr. 2015;61:491–8.
Ehrenkranz RA. Early nutritional support and outcomes in ELBW infants. Early Hum Dev. 2010;86:21–5.
Bonsante F, Iacobelli S, Latorre G, Rigo J, de Felice C, Robillard PY, et al. Initial amino acid intake influences phosphorus and calcium homeostasis in preterm infants - it is time to change the composition of the early parenteral nutrition. PLoS ONE. 2013;8:1–9.
Moltu SJ, Strømmen K, Blakstad EW, Almaas AN, Westerberg AC, Brække K, et al. Enhanced feeding in very-low-birth-weight infants may cause electrolyte disturbances and septicemia–a randomized, controlled trial. Clin Nutr. 2013;32:207–12.
Craddock PR, Yawata Y, VanSanten L, Gilberstadt S, Silvis S, Jacob HS. Acquired phagocyte dysfunction. A complication of the hypophosphatemia of parenteral hyperalimentation. N Engl J Med. 1974;290:1403–7.
Brener Dik PH, Galletti MF, Fernández Jonusas SA, Alonso G, Mariani GL, Fustiñana CA. Early hypophosphatemia in preterm infants receiving aggressive parenteral nutrition. J Perinatol. 2015;35:712–5.
Ross JR, Finch C, Ebeling M, Taylor SN. Refeeding syndrome in very-low-birth-weight intrauterine growth-restricted neonates. J Perinatol. 2013;33:717–20.
Bustos Lozano G, Hidalgo Romero Á, Melgar Bonis A, Ureta Velasco N, Orbea Gallardo C, Pallás, et al. Early hypophosphataemia in at risk newborns. Frequency Magnit Pediatr. 2018;88:216–22.
Yang SM, Noh OK, Lee JH, Park MS. Late and insufficient phosphorus supplementation is associated with early severe hypophosphatemia in extremely low birth weight infants with early amino acid administration. Perinatology. 2018;29:13.
Trivedi A, Sinn John KH. Early versus late administration of amino acids in preterm infants receiving parenteral nutrition. Cochrane Database Syst Rev. 2013. http://onlinelibrary.wiley.com/doi/10.1002/14651858.CD008771.pub2/abstract.
Bustos Lozano G, Soriano-Ramos M, Pinilla Martín MT, Chumillas Calzada S, García Soria CE, Pallás-Alonso CR. Early hypophosphatemia in high-risk preterm infants: efficacy and safety of sodium glycerophosphate from first day on parenteral nutrition. J Parenter Enter Nutr. 2019;43:419–25.
Pająk A, Królak-Olejnik B, Szafrańska A. Early hypophosphatemia in very low birth weight preterm infants. Adv Clin Exp Med. 2018;27:841–7.
Senterre J, Salle B. Renal aspects of calcium and phosphorus metabolism in preterm infants. Biol Neonate. 1988;53:220–9.
Torrazza RM, Neu J. Evidence-based guidelines for optimization of nutrition for the very low birthweight infant. Neoreviews. 2013;14:e340–9.
Ichikawa G, Watabe Y, Suzumura H, Sairenchi T, Muto T, Arisaka O. Hypophosphatemia in small for gestational age extremely low birth weight infants receiving parenteral nutrition in the first week after birth. J Pediatr Endocrinol Metab. 2012;25:317–21.
Vileisis RA. Effect of phosphorous intake in total parenteral nutrition infusates in premature neonates. J Pediatr. 1987;110:586–90.
Moe K, Beck-Nielsen SS, Lando A, Greisen G, Zachariassen G. Administering different levels of parenteral phosphate and amino acids did not influence growth in extremely preterm infants. Acta Paediatr. 2015;104:894–9.
Mihatsch W, Fewtrell M, Goulet O, Molgaard C, Picaud JC, Senterre T, et al. ESPGHAN/ESPEN/ESPR/CSPEN guidelines on pediatric parenteral nutrition: calcium, phosphorus and magnesium. Clin Nutr. 2018;37:2360–5.
Johnson PJ. Review of micronutrients in parenteral nutrition for the NICU population. Neonatal Netw. 2014;33:155–61.
Mulla S, Stirling S, Cowey S, Close R, Pullan S, Howe R, et al. Severe hypercalcaemia and hypophosphataemia with an optimised preterm parenteral nutrition formulation in two epochs of differing phosphate supplementation. Arch Dis Child Fetal Neonatal Ed. 2017;102:F451–5.
Boubred F, Herlenius E, Bartocci M, Jonsson B, Vanpée M. Extremely preterm infants who are small for gestational age have a high risk of early hypophosphatemia and hypokalemia. Acta Paediatr. 2015;104:1077–83.
Mena Nannig P, Cubillos Celis MP, Toro Jara C, Zuñiga Vergara C. Prematuro Extremo - Perfil Bioquímico en Sangre Cordón y Crecimiento Fetal. Rev Chil Pediatr. 2016;87:250–4.
Author information
Authors and Affiliations
Contributions
MFG was responsible for designing and writing the protocol, conducting the search, extracting, analyzing data, interpreting results, drafted the initial manuscript, and approved the final manuscript as submitted. PHBD contributed to the statistical analysis of data, revised the manuscript, and approved the final manuscript as submitted. SFJA aided in the design of the study, reviewed, and revised the manuscript, and approved the final manuscript as submitted. DS, CC, SP, MB, GL, and IA coordinated the study in their Institutions and contributed to the acquisition of patient’s data. They also revised the manuscript and approved the final manuscript as submitted. GLM mentored MFG in study conception, critically reviewed the manuscript, and approved the final manuscript as submitted.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Galletti, M.F., Brener Dik, P.H., Fernandez Jonusas, S.A. et al. Early high amino-acid intake is associated with hypophosphatemia in preterm infants. J Perinatol 42, 1063–1069 (2022). https://doi.org/10.1038/s41372-022-01361-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41372-022-01361-1