Abstract
Objective
To compare 2% aqueous chlorhexidine gluconate (AQC) vs. 2% chlorhexidine gluconate in 70% isopropyl alcohol (ALC) for pre-venipuncture skin antisepsis in very-low-birth-weight neonates (VLBW, birth-weight <1500 grams).
Study design
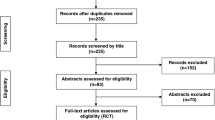
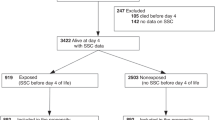
Double-blind, non-inferiority trial randomized 199 VLBW neonates, age 2–28 days, to receive pre-venipuncture skin preparation using single application of swabstick impregnated with AQC (n = 99) or ALC (n = 100). Skin clearance rate (percentage post-cleansing skin swabs with <15 bacterial colony forming units) with a 10% non-inferiority margin for AQC was primary outcome. Absolute and relative CFU reduction and adverse skin reactions were compared.
Results
AQC’s clearance was non-inferior to ALC (91% vs. 88%; 95% CI −6.6%, +12.4%). Median (interquartile range) absolute [61 (16, 110) vs. 63 (18, 100); p = 0.65] and relative [100% (97%, 100%) vs. 100% (99.7%, 100%); p = 0.20] CFU reductions were similar. Neither group experienced any adverse reactions.
Conclusion
AQC may provide non-inferior skin disinfection to ALC in VLBW neonates.
Clinical Trial registration
ClinicalTrials.gov NCT01270776.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
Data availability
Individual de-identified participant data that underlie the results reported in this manuscript and the study protocol will be available, pending approval by the institutional research ethics board, immediately following publication, for investigators who provide a methodologically sound proposal approved by an independent review committee, to achieve aims cited in the approved proposal. Data request and proposals should be directed to the corresponding author. Requestors will need to sign a data sharing agreement.
References
Frasca D, Dahyot-Fizelier C, Mimoz O. Prevention of central venous catheter-related infection in the intensive care unit. Crit Care. 2010;14:212.
Visscher MO, Adam R, Brink S, Odio M. Newborn infant skin: physiology, development, and care. Clin Dermatol. 2015;33:271–80.
D’Angio CT, McGowan KL, Baumgart S, St Geme J, Harris MC. Surface colonization with coagulase-negative staphylococci in premature neonates. J Pediatr. 1989;114:1029–34.
Keyworth N, Millar MR, Holland KT. Development of cutaneous microflora in premature neonates. Arch Dis Child. 1992;67:797–801.
Courtois E, Cimerman P, Dubuche V, Goiset MF, Orfevre C, Lagarde A, et al. The burden of venipuncture pain in neonatal intensive care units: EPIPPAIN 2, a prospective observational study. Int J Nurs Stud. 2016;57:48–59.
Heron TJ, Faraday CM, Clarke P. The hidden harms of Matching Michigan. Arch Dis Child Fetal Neonatal Ed. 2013;98:F466–7.
Payne V, Hall M, Prieto J, Johnson M. Care bundles to reduce central line-associated bloodstream infections in the neonatal unit: a systematic review and meta-analysis. Arch Dis Child Fetal Neonatal Ed. 2018;103:F422–9.
O’Grady NP, Alexander M, Dellinger EP, Gerberding JL, Heard SO, Maki DG, et al. Guidelines for the prevention of intravascular catheter-related infections. Centers for disease control and prevention. MMWR Recommendations Rep: Morbidity Mortal Wkly Rep. Recommendations Rep. 2002;51:1–29.
Pratt RJ, Pellowe CM, Wilson JA, Loveday HP, Harper PJ, Jones SR, et al. epic2: National evidence-based guidelines for preventing healthcare-associated infections in NHS hospitals in England. J Hospital Infect. 2007;65:S1–64.
Loveday HP, Wilson JA, Pratt RJ, Golsorkhi M, Tingle A, Bak A, et al. epic3: national evidence-based guidelines for preventing healthcare-associated infections in NHS hospitals in England. J Hospital Infect. 2014;86:S1–70.
Ponnusamy V, Venkatesh V, Clarke P. Skin antisepsis in the neonate: what should we use? Curr Opin Infect Dis. 2014;27:244–50.
Sathiyamurthy S, Banerjee J, Godambe SV. Antiseptic use in the neonatal intensive care unit - a dilemma in clinical practice: An evidence based review. World J Clin Pediatr. 2016;5:159–71.
Datta MK, Clarke P. Current practices in skin antisepsis for central venous catheterisation in UK tertiary-level neonatal units. Arch Dis Child Fetal Neonatal Ed. 2008;93:F328.
Tamma PD, Aucott SW, Milstone AM. Chlorhexidine use in the neonatal intensive care unit: results from a national survey. Infect control Hospital Epidemiol: Off J Soc Hospital Epidemiologists Am. 2010;31:846–9.
Johnson J, Bracken R, Tamma PD, Aucott SW, Bearer C, Milstone AM. Trends in chlorhexidine use in US neonatal intensive care units: results from a follow-up national survey. Infect control Hospital Epidemiol: Off J Soc Hospital Epidemiologists Am. 2016;37:1116–8.
Keyworth N, Millar MR, Holland KT. Swab-wash method for quantitation of cutaneous microflora. J Clin Microbiol. 1990;28:941–3.
Garland JS, Alex CP, Uhing MR, Peterside IE, Rentz A, Harris MC. Pilot trial to compare tolerance of chlorhexidine gluconate to povidone-iodine antisepsis for central venous catheter placement in neonates. J Perinatol. 2009;29:808–13.
Maki DG, Ringer M. Evaluation of dressing regimens for prevention of infection with peripheral intravenous catheters. Gauze, a transparent polyurethane dressing, and an iodophor-transparent dressing. JAMA: J Am Med Assoc. 1987;258:2396–403.
Lilley CPA, Gray A. A prospective randomised double blind Comparison of 0.5% versus 0.05% aqueous Chlorhexidine for skin antisepsis prior to line insertion in neonates (abstract). Arch Dis Child. 2006;91:A17–9.
Chaiyakunapruk N, Veenstra DL, Lipsky BA, Saint S. Chlorhexidine compared with povidone-iodine solution for vascular catheter-site care: a meta-analysis. Ann Intern Med. 2002;136:792–801.
Garland JS, Buck RK, Maloney P, Durkin DM, Toth-Lloyd S, Duffy M, et al. Comparison of 10% povidone-iodine and 0.5% chlorhexidine gluconate for the prevention of peripheral intravenous catheter colonization in neonates: a prospective trial. Pediatr Infect Dis J. 1995;14:510–6.
Garland JS, Alex CP, Mueller CD, Otten D, Shivpuri C, Harris MC, et al. A randomized trial comparing povidone-iodine to a chlorhexidine gluconate-impregnated dressing for prevention of central venous catheter infections in neonates. Pediatrics. 2001;107:1431–6.
Garland JS, Alex CP, Mueller CD, Cisler-Kahill LA. Local reactions to a chlorhexidine gluconate-impregnated antimicrobial dressing in very low birth weight infants. Pediatr Infect Dis J. 1996;15:912–4.
Valles J, Fernandez I, Alcaraz D, Chacon E, Cazorla A, Canals M, et al. Prospective randomized trial of 3 antiseptic solutions for prevention of catheter colonization in an intensive care unit for adult patients. Infect control Hospital Epidemiol: Off J Soc Hospital Epidemiologists Am. 2008;29:847–53.
Sharma A, Kulkarni S, Thukral A, Sankar MJ, Agarwal R, Deorari AK, et al. Aqueous chlorhexidine 1% versus 2% for neonatal skin antisepsis: a randomised non-inferiority trial. Arch Dis Child Fetal Neonatal Ed. 2021;106:643–8.
Clarke P, Craig JV, Wain J, Tremlett C, Linsell L, Bowler U, et al. Safety and efficacy of 2% chlorhexidine gluconate aqueous versus 2% chlorhexidine gluconate in 70% isopropyl alcohol for skin disinfection prior to percutaneous central venous catheter insertion in preterm neonates: the ARCTIC randomised-controlled feasibility trial protocol. BMJ Open. 2019;9:e028022.
Shah D, Tracy M. Skin antisepsis survey in Australia-New Zealand neonatal nurseries. J paediatrics child health. 2013;49:601–2.
Vanzi V, Pitaro R. Skin injuries and chlorhexidine gluconate-based antisepsis in early premature infants: a case report and review of the literature. J Perinat neonatal Nurs. 2018;32:341–50.
Funding
The authors (AJ, VS) received peer-reviewed operating grant from the Physicians’ Services Incorporated (PSI) Foundation, Ontario, Canada, which supported all phases of this study. The funding agency had no role in study design, collection, analysis or interpretation of data, writing this manuscript or the decision to submit it for publication.
Author information
Authors and Affiliations
Contributions
AJ designed and conceptualized the study, obtained the operating grant to support the study, carried out study procedures, coordinated and assisted in patient recruitment, supervised data collection, prepared the initial draft of the manuscript, and reviewed and revised the final manuscript. PD contributed in designing the study, carried out study procedures, coordinated and assisted in patient recruitment and data collection, and reviewed and revised the final manuscript. WY assisted by designing the statistical section of the study proposal, contributed in the successful grant proposal to obtain operating funds, conducted the interim as well as the final analysis for the study, and reviewed and revised the final manuscript. KSL was the site investigator for the secondary study site. KSL was directly responsible for obtaining local approvals, and coordinating and supervising recruitment and data collection at secondary study site, and reviewed and revised the final manuscript. AM contributed by designing the microbiology methodology for the study proposal as well as for the successful grant proposal to obtain operating funds, supervised microbiology staff in analyzing and reporting study specimens, and reviewed and revised the final manuscript. VS designed and conceptualized the study, obtained the operating grant to support the study, provided overall senior supervision in the day-to-day conduct of the study, and reviewed and revised the final manuscript. All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval
The study was approved by institutional research ethics board at both study sites and informed consent was obtained from all participants before inclusion.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The study was presented at an abstract at the Pediatric Academic Society’s (PAS) annual conference, 2014.
Rights and permissions
About this article
Cite this article
Jain, A., Deshpande, P., Yoon, E.W. et al. 2% aqueous vs alcohol-based chlorhexidine for skin antisepsis in VLBW neonates undergoing peripheral venipuncture: a non-inferiority trial. J Perinatol 42, 636–641 (2022). https://doi.org/10.1038/s41372-022-01337-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41372-022-01337-1