Objectives
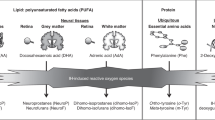
Premature neonates often receive oral sucrose or dextrose before tissue-damaging procedures (TDPs). Previous work showed that a single dose of sucrose, but not dextrose, increased cellular energy utilization and ATP degradation. This pilot study probes the effects of repeated administration of sucrose or dextrose on energy metabolism.
Methods
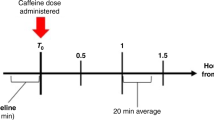
Urinary markers of ATP metabolism (hypoxanthine, xanthine, uric acid) are measured in premature neonates randomized to receive: (a) standard of care, (b) 0.2 ml 24% oral sucrose, or (c) 0.2 ml 30% oral dextrose, before every painful procedure on days-of-life 3–7.
Results
Standard of care is associated with highest xanthine/creatinine and uric acid/creatinine, likely because of fewer pain treatments. Benefits of repeated oral sucrose are unclear. Neonates receiving oral dextrose had lower xanthine/creatinine and uric acid/creatinine.
Conclusions
Repeated treatments of neonatal procedural pain with 30% oral dextrose are less energetically demanding. Larger clinical studies are needed for comparison with sucrose treatments.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Cruz MD, Fernandes AM, Oliveira CR. Epidemiology of painful procedures performed in neonates: a systematic review of observational studies. Eur J Pain. 2016;20:489–98.
Sen E, Manav G. Effect of Kangaroo care and oral sucrose on pain in premature infants: a randomized controlled trial. Pain Manag Nurs. 2020;21:556–64.
Ranjbar A, Bernstein C, Shariat M, Ranjbar H. Comparison of facilitated tucking and oral dextrose in reducing the pain of heel stick in preterm infants: a randomized clinical trial. BMC Pediatr. 2020;20:162.
Matsuda E. Sucrose as analgesia in neonates undergoing painful procedures. Am J Nurs. 2017;117:21.
Asmerom Y, Slater L, Boskovic DS, Bahjri K, Holden MS, Phillips R, et al. Oral sucrose for heel lance increases adenosine triphosphate use and oxidative stress in preterm neonates. J Pediatr. 2013;163:29–35 e1.
Angeles DM, Asmerom Y, Boskovic DS, Slater L, Bacot-Carter S, Bahjri K, et al. Oral sucrose for heel lance enhances adenosine triphosphate use in preterm neonates with respiratory distress. SAGE Open Med. 2015;3:2050312115611431.
Angeles DM, Boskovic DS, Tan JC, Shih W, Hoch E, Forde D, et al. Oral dextrose reduced procedural pain without altering cellular ATP metabolism in preterm neonates: a prospective randomized trial. J Perinatol. 2020;40:888–95.
Liemburg-Apers DC, Imamura H, Forkink M, Nooteboom M, Swarts HG, Brock R, et al. Quantitative glucose and ATP sensing in mammalian cells. Pharm Res. 2011;28:2745–57.
Zhang DM, Li YC, Xu D, Ding XQ, Kong LD. Protection of curcumin against fructose-induced hyperuricaemia and renal endothelial dysfunction involves NO-mediated JAK-STAT signalling in rats. Food Chem. 2012;134:2184–93.
Holden MS, Hopper A, Slater L, Asmerom Y, Esiaba I, Boskovic DS, et al. Urinary hypoxanthine as a measure of increased ATP utilization in late preterm infants. Infant Child Adolesc Nutr. 2014;6:240–9.
Reuter S, Moser C, Baack M. Respiratory distress in the newborn. Pediatr Rev. 2014;35:417–28.
Turgan N, Boydak B, Habif S, Gulter C, Senol B, Mutaf I, et al. Urinary hypoxanthine and xanthine levels in acute coronary syndromes. Int J Clin Lab Res. 1999;29:162–5.
Esiaba I, Angeles DM, Holden MS, Tan JB, Asmerom Y, Gollin G, et al. Urinary allantoin is elevated in severe intraventricular hemorrhage in the preterm newborn. Transl Stroke Res. 2016;7:97–102.
Forde D, Deming DD, Tan JC, Phillips RM, Fry-Bowers EK, Barger MK, et al. Oxidative stress biomarker decreased in preterm neonates treated with Kangaroo mother care. Biol Res Nurs. 2020;22:188–96.
George SK, Dipu MT, Mehra UR, Singh P, Verma AK, Ramgaokar JS. Improved HPLC method for the simultaneous determination of allantoin, uric acid and creatinine in cattle urine. J Chromatogr B Anal Technol Biomed Life Sci. 2006;832:134–7.
Slater L, Asmerom Y, Boskovic DS, Bahjri K, Plank MS, Angeles KR, et al. Procedural pain and oxidative stress in premature neonates. J Pain. 2012;13:590–7.
Martini S, Austin T, Aceti A, Faldella G, Corvaglia L. Free radicals and neonatal encephalopathy: mechanisms of injury, biomarkers, and antioxidant treatment perspectives. Pediatr Res. 2020;87:823–33.
Plank MS, Calderon TC, Asmerom Y, Boskovic DS, Angeles DM. Biochemical measurement of neonatal hypoxia. J Vis Exp. 2011;54:e2948.
Plank MS, Boskovic DS, Tagge E, Chrisler J, Slater L, Angeles KR, et al. An animal model for measuring the effect of common NICU procedures on ATP metabolism. Biol Res Nurs. 2011;13:283–8.
Young JD, Yao SY, Baldwin JM, Cass CE, Baldwin SA. The human concentrative and equilibrative nucleoside transporter families, SLC28 and SLC29. Mol Asp Med. 2013;34:529–47.
Frenguelli BG, Dale N. Purines: from diagnostic biomarkers to therapeutic agents in brain injury. Neurosci Bull. 2020;36:1315–26.
Acknowledgements
We thank Yayesh Asmerom MS, for performing all the purine measurements and Desiree Wallace, PharmD, for providing the study drug and assisting with the randomization of subjects. We thank Judy Gates, RN, MS for subject enrollment and Cassia Owen MS for assisting with sample collection and preparation. We also thank the NICU nurses at Loma Linda University Children’s Hospital for their support of this project.
Funding
This work was supported by NIH grant RO1 NR011209 (Angeles).
Author information
Authors and Affiliations
Contributions
All authors provided final approval of the submitted final version of the paper. DA designed the experiments, acquired the funding, participated in the analysis and interpretation of the data, wrote the original and final draft, and is accountable for all aspects of the study. DB participated in the interpretation of the data, made substantial contributions to the writing of the paper, and is accountable for all aspects of the study. EF assisted in the design of the study and acquisition of data, participated in the interpretation of the data, made substantial contributions to the paper, and is accountable for all aspects of the study. KB participated in the analysis and interpretation of the data and made substantial contributions to the writing of the paper and is accountable for all aspects of the study. DD, AH, RP, AC, EH, RP, JT, VC, PP, MG, GT participated in the acquisition and interpretation of the data, made substantial contributions to the writing of the paper and are accountable for all aspects of the study.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Angeles, D.M., Boskovic, D.S., Deming, D. et al. A pilot study on the biochemical effects of repeated administration of 24% oral sucrose vs. 30% oral dextrose on urinary markers of adenosine triphosphate degradation. J Perinatol 41, 2761–2765 (2021). https://doi.org/10.1038/s41372-021-01239-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41372-021-01239-8