Abstract
Objective
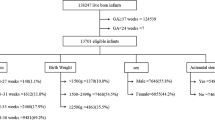
To compare the difference in outcomes in a subset population of infants “eligible but not enrolled; ENE” vs those who were “eligible and enrolled, EE” in The Australian Placental Transfusion Study (APTS).
Study design
Population-based multicentre retrospective cohort study.
Results
A total of 535 (17.7%) infants were categorized as EE and 2489 (82.3%) ENE. ENE infants were significantly more premature (mean gestation 27.0 vs 28.0 weeks) but otherwise of similar anthropometric measures compared to EE infants. ENE infants had significantly higher incidences of low Apgar scores <7 at 5 min, CLD, IVH and PDA requiring treatment. Using a multivariate adjusted-analysis, ENE were at a greater risk for mortality (OR 1.86; 95% CI, 1.30–2.67, p < 0.001).
Conclusion
Antenatal consenting may lead to biased population representation, which may affect trial results’ generalizability. Retrospective consent or waiver of consent may improve the generalizability of neonatal and emergency clinical trials.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
Data availability
Data are available on reasonable request. All data relevant to the study are included in the article. All data were extracted and supplied by NICUS and are available from the corresponding author on reasonable request and with permission of the study team and NICUS Data Management Committee.
References
Eltorki M, Uleryk E, Freedman SB. Waiver of informed consent in pediatric resuscitation research: a systematic review. Acad Emerg Med. 2013;20:822–34.
Rich W, Finer NN, Gantz MG, Newman NS, Hensman AM, Hale EC, et al. Enrollment of extremely low birth weight infants in a clinical research study may not be representative. Pediatrics. 2012;129:480–4.
Mason S. Obtaining informed consent for neonatal randomised controlled trials-an “elaborate ritual”? Arch Dis Child Fetal Neonatal Ed. 1997;76:F143–145.
Burgess E, Singhal N, Amin H, McMillan DD, Devrome H. Consent for clinical research in the neonatal intensive care unit: a retrospective survey and a prospective study. Arch Dis Child Fetal Neonatal Ed. 2003;88:F280–285.
Support Study Group of the Eunice Kennedy Shriver NICHD Neonatal Research Network, Carlo WA, Finer NN, Walsh MC, Rich W, Gantz MG, et al. Target ranges of oxygen saturation in extremely preterm infants. N Engl J Med. 2010;362:1959–69.
Support Study Group of the Eunice Kennedy Shriver NICHD Neonatal Research Network, Finer NN, Carlo WA, Walsh MC, Rich W, Gantz MG, et al. Early CPAP versus surfactant in extremely preterm infants. N Engl J Med. 2010;362:1970–9.
Roberts CT, Owen LS, Manley BJ, Froisland DH, Donath SM, Dalziel KM, et al. Nasal high-flow therapy for primary respiratory support in preterm infants. N Engl J Med. 2016;375:1142–51.
Songstad NT, Roberts CT, Manley BJ, Owen LS, Davis PG, HIPSTER trial investigators. Retrospective consent in a neonatal randomized controlled trial. Pediatrics. 2018;141:e20172092.
Tarnow-Mordi W, Morris J, Kirby A, Robledo K, Askie L, Brown R, et al. Delayed versus immediate cord clamping in preterm infants. N Engl J Med. 2017;377:2445–55.
Janz-Robinson EM, Badawi N, Walker K, Bajuk B, Abdel-Latif ME, Neonatal Intensive Care Units N. Neurodevelopmental outcomes of premature infants treated for patent ductus arteriosus: a population-based cohort study. J Pediatr. 2015;167:1025–1032 e1023.
Papile LA, Burstein J, Burstein R, Koffler H. Incidence and evolution of subependymal and intraventricular hemorrhage: a study of infants with birth weights less than 1,500 gm. J Pediatr. 1978;92:529–34.
Patz A. New international classification of retinopathy of prematurity. Pediatrics. 1984;74:160–1.
Lawrence G, Tudehope D, Baumann K, Jeffery H, Gill A, Cole M, et al. Enteral human IgG for prevention of necrotising enterocolitis: a placebo-controlled, randomised trial. Lancet. 2001;357:2090–4.
Bell MJ, Ternberg JL, Feigin RD, Keating JP, Marshall R, Barton L, et al. Neonatal necrotizing enterocolitis. Therapeutic decisions based upon clinical staging. Ann Surg. 1978;187:1–7.
NSW and ACT Neonatal Intensive Care Units Audit Group. Neonatal Intensive Care Units’ (NICUS) Data Registry 2020. 2020.
Davis S, Wright PW, Schulman SF, Hill LD, Pinkham RD, Johnson LP, et al. Participants in prospective, randomized clinical trials for resected non-small cell lung cancer have improved survival compared with nonparticipants in such trials. Cancer. 1985;56:1710–8.
Schmidt B, Gillie P, Caco C, Roberts J, Roberts R. Do sick newborn infants benefit from participation in a randomized clinical trial? J Pediatr. 1999;134:151–5.
Ayers S, Sawyer A, During C, Rabe H. Parents report positive experiences about enrolling babies in a cord-related clinical trial before birth. Acta Paediatr. 2015;104:e164–170.
Culbert A, Davis DJ. Parental preferences for neonatal resuscitation research consent: a pilot study. J Med Ethics. 2005;31:721–6.
Rich WD, Katheria AC. Waiver of consent in a trial intervention occurring at birth-how do parents feel? Front Pediatr. 2017;5:56.
Luce JM. Is the concept of informed consent applicable to clinical research involving critically ill patients? Crit Care Med. 2003;31:S153–160. 3 Suppl
Truog RD, Robinson W, Randolph A, Morris A. Is informed consent always necessary for randomized, controlled trials? N. Engl J Med. 1999;340:804–7.
World Medical A. World Medical Association Declaration of Helsinki. Ethical principles for medical research involving human subjects. Bull World Health Organ. 2001;79:373–4.
Stenson BJ, Becher JC, McIntosh N. Neonatal research: the parental perspective. Arch Dis Child Fetal Neonatal Ed. 2004;89:F321–323.
Acknowledgements
The authors thank the Directors, the NICUS members and the audit officers of all tertiary units in supporting this collaborative study: NICUS, Dr Himanshu Popat (Chairperson), Barbara Bajuk (Coordinator), Sara Sedgley (CNS); Canberra Hospital, Dr Hazel Carlisle (Director), Melanie Rosin; John Hunter Children’s Hospital, Dr Larissa Korostenski (Director), Alissa Argomand, Stacey Leonard; Royal Prince Alfred Hospital, Dr Mark Greenhalgh (Director), Shelley Reid; Liverpool Hospital, Dr Jacqueline Stack (Director), Dr Ian Callander, Kathryn Medlin, Amanda Beesley; Nepean Hospital, Dr Lyn Downe, Mee Fong Chin; The Children’s Hospital at Westmead, Prof Nadia Badawi (Director), Karina Rogers; Royal North Shore Hospital, Dr Mary Paradisis (Director), Prof Martin Kluckow, Lyn Barnes; Sydney Children’s Hospital, Dr Andrew Numa (Director), Dr Gary Williams, Janelle Young; Westmead Hospital, Dr Melissa Luig (Director), Jane Baird, Gemma Lowe; and Royal Hospital for Women, Dr Srinivas Bolisetty (Director), Prof Julee Oei, Diane Cameron. We also thank the babies and their families, the nursing and midwifery, obstetric and medical records staff of the obstetric and children’s hospitals in NSW and the ACT. The authors also thank the Australian Placental Transfusion Study Collaborative Group. Special thanks to Professor William Tarnow-Mordi, Dr Kristy Robledo, Ms. Carbo Yeung, and Mr. Syamand Hasam for helping with the data extraction and commenting on the research proposal.
Author information
Authors and Affiliations
Contributions
AS carried out the statistical analyses, contributed to the data interpretation, drafted the initial manuscript, and reviewed and revised the manuscript. BB contributed to study design, retrieved and cleaned the data, contributed to the data interpretation, and reviewed and revised the manuscript. MEA conceptualized and designed the study, carried out the statistical analyses, contributed to the data interpretation, led the writing, and reviewed and revised the manuscript. All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics statements
Health Human Research Ethics Committee (2019.LRE.00234) has approved this study. Patient consent for publication was not required. The study was performed in accordance with the Declaration of Helsinki.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Shastry, A., Bajuk, B. & Abdel-Latif, M.E. Are we enrolling representative cohorts of premature infants in our clinical trials?. J Perinatol 42, 86–90 (2022). https://doi.org/10.1038/s41372-021-01204-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41372-021-01204-5