Abstract
Objective
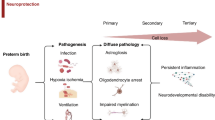
To assess the feasibility and safety of one dose of Darbepoetin alpha (Darbe) administered to neonates ≥34 weeks with mild neonatal encephalopathy (NE).
Methods
Randomized, masked, placebo-controlled study including neonates ≥34 weeks gestation with mild NE. Neonates were randomized to receive one dose of Darbe (10 μg/kg IV) or placebo. Clinical and laboratory maternal and newborn data were collected. The Bayley Scales of Infant and Toddler Development, third edition (Bayley-III) and a standardized neurological examination at 8–12 months of corrected age were assessed.
Results
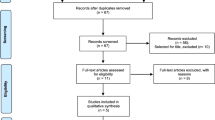
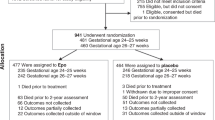
There were no differences in baseline characteristics of the 21 infants randomized (9 Darbe, 12 placebo). Adverse events were not reported at any time. Bayley-III scores were average in both Darbe and placebo groups.
Conclusion
This study demonstrates that a randomized, masked, placebo-controlled trial is safe and feasible. A large, randomized trial is warranted to assess the effect of Darbe in this population.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
DuPont TL, Chalak LF, Morriss MC, Burchfield PJ, Christie L, Sánchez PJ. Short-term outcomes of newborns with perinatal acidemia who are not eligible for systemic hypothermia therapy. J Pediatr. 2013;162:35–41.
Kracer B, Hintz SR, Van Meurs KP, Lee HC. Hypothermia therapy for neonatal hypoxic ischemic encephalopathy in the state of California. J Pediatr. 2014;165:267–73.
Gagne-Loranger M, Sheppard M, Ali N, Saint-Martin C, Wintermark P. Newborns referred for therapeutic hypothermia: association between initial degree of encephalopathy and severity of brain injury (what about the newborns with mild encephalopathy on admission?). Am J Perinatol. 2016;33:195–202.
Prempunpong C, Chalak LF, Garfinkle J, Shah B, Kalra V, Rollins N, et al. Prospective research on infants with mild encephalopathy: the PRIME study. J Perinatol J Calif Perinat Assoc. 2017;38:80–85.
Sarnat HB, Sarnat MS. Neonatal encephalopathy following fetal distress. A clinical and electroencephalographic study. Arch Neurol. 1976;33:696–705.
Finer NN, Robertson CM, Richards RT, Pinnell LE, Peters KL. Hypoxic-ischemic encephalopathy in term neonates: perinatal factors and outcome. J Pediatr. 1981;98:112–7.
Murray DM, O’Connor CM, Ryan CA, Korotchikova I, Boylan GB. Early EEG grade and outcome at 5 years after mild neonatal hypoxic ischemic encephalopathy. Pediatrics. 2016;138:e20160659.
Walsh BH, Neil J, Morey J, Yang E, Silvera MV, Inder TE, et al. The frequency and severity of magnetic resonance imaging abnormalities in infants with mild neonatal encephalopathy. J Pediatr. 2017;187:26–33.
Chalak LF, Nguyen K-A, Prempunpong C, Heyne R, Thayyil S, Shankaran S, et al. Prospective research in infants with mild encephalopathy identified in the first six hours of life: neurodevelopmental outcomes at 18–22 months. Pediatr Res. 2018;84:861–8.
Massaro AN, Murthy K, Zaniletti I, Cook N, DiGeronimo R, Dizon M, et al. Short-term outcomes after perinatal hypoxic ischemic encephalopathy: a report from the Children’s Hospitals Neonatal Consortium HIE focus group. J Perinatol J Calif Perinat Assoc. 2015;35:290–6.
Dame C, Bartmann P, Wolber E, Fahnenstich H, Hofmann D, Fandrey J. Erythropoietin gene expression in different areas of the developing human central nervous system. Brain Res Dev Brain Res. 2000;125:69–74.
Jantzie LL, Getsy PM, Firl DJ, Wilson CG, Miller RH, Robinson S. Erythropoietin attenuates loss of potassium chloride co-transporters following prenatal brain injury. Mol Cell Neurosci. 2014;61:152–62.
Jantzie LL, Corbett CJ, Firl DJ, Robinson S. Postnatal erythropoietin mitigates impaired cerebral cortical development following subplate loss from prenatal hypoxia-ischemia. Cereb Cortex. 2015;25:2683–95.
Matsushita H, Johnston MV, Lange MS, Wilson MA. Protective effect of erythropoietin in neonatal hypoxic ischemia in mice. Neuroreport. 2003;14:1757–61.
Shingo T, Sorokan ST, Shimazaki T, Weiss S. Erythropoietin regulates the in vitro and in vivo production of neuronal progenitors by mammalian forebrain neural stem cells. J Neurosci J Soc Neurosci. 2001;21:9733–43.
Sirén AL, Fratelli M, Brines M, Goemans C, Casagrande S, Lewczuk P, et al. Erythropoietin prevents neuronal apoptosis after cerebral ischemia and metabolic stress. Proc Natl Acad Sci USA. 2001;98:4044–9.
Iwai M, Cao G, Yin W, Stetler RA, Liu J, Chen J. Erythropoietin promotes neuronal replacement through revascularization and neurogenesis after neonatal hypoxia/ischemia in rats. Stroke. 2007;38:2795–803.
Banks WA, Jumbe NL, Farrell CL, Niehoff ML, Heatherington AC. Passage of erythropoietic agents across the blood-brain barrier: a comparison of human and murine erythropoietin and the analog Darbepoetin alfa. Eur J Pharm. 2004;505:93–101.
Baserga MC, Beachy JC, Roberts JK, Ward RM, DiGeronimo RJ, Walsh WF, et al. Darbepoetin administration to neonates undergoing cooling for encephalopathy: a safety and pharmacokinetic trial. Pediatr Res. 2015;78:315–22.
Shankaran S, Laptook AR, Ehrenkranz RA, Tyson JE, McDonald SA, Donovan EF, et al. Whole-body hypothermia for neonates with hypoxic-ischemic encephalopathy. N Engl J Med. 2005;353:1574–84.
Chalak LF, Adams-Huet B, Sant’Anna G. A total sarnat score in mild hypoxic-ischemic encephalopathy can detect infants at higher risk of disability. J Pediatr. 2019;214:217.e1.
Finder M, Boylan GB, Twomey D, Ahearne C, Murray DM, Hallberg B. Two-year neurodevelopmental outcomes after mild hypoxic ischemic encephalopathy in the era of therapeutic hypothermia. JAMA Pediatr. 2020;174:48–55.
Wang B, Armstrong JS, Reyes M, Kulikowicz E, Lee J-H, Spicer D, et al. White matter apoptosis is increased by delayed hypothermia and rewarming in a neonatal piglet model of hypoxic ischemic encephalopathy. Neuroscience. 2016;316:296–310.
O’Brien CE, Santos PT, Kulikowicz E, Reyes M, Koehler RC, Martin LJ, et al. Hypoxia-ischemia and hypothermia independently and interactively affect neuronal pathology in neonatal piglets with short-term recovery. Dev Neurosci. 2019;41:17–33.
Rao R, Trivedi S, Distler A, Liao S, Vesoulis Z, Smyser C, et al. Neurodevelopmental outcomes in neonates with mild hypoxic ischemic encephalopathy treated with therapeutic hypothermia. Am J Perinatol. 2019;36:1337–43.
Kirkley MJ, Boohaker L, Griffin R, Soranno DE, Gien J, Askenazi D, et al. Acute kidney injury in neonatal encephalopathy: an evaluation of the AWAKEN database. Pediatr Nephrol Berl Ger. 2019;34:169–76.
Berube MW, Lemmon ME, Pizoli CE, Bidegain M, Tolia VN, Cotten CM, et al. Opioid and benzodiazepine use during therapeutic hypothermia in encephalopathic neonates. J Perinatol. 2019;280:51–61.
Tata DA, Markostamou I, Ioannidis A, Gkioka M, Simeonidou C, Anogianakis G, et al. Effects of maternal separation on behavior and brain damage in adult rats exposed to neonatal hypoxia–ischemia. Behav Brain Res. 2015;280:51–61.
Lemmon ME, Donohue PK, Parkinson C, Northington FJ, Boss RD. Parent experience of neonatal encephalopathy. J Child Neurol. 2017;32:286–92.
Gonzalez FF, McQuillen P, Mu D, Chang Y, Wendland M, Vexler Z, et al. Erythropoietin enhances long-term neuroprotection and neurogenesis in neonatal stroke. Dev Neurosci. 2007;29:321–30.
Martinez-Biarge M, Diez-Sebastian J, Wusthoff CJ, Lawrence S, Aloysius A, Rutherford MA, et al. Feeding and communication impairments in infants with central grey matter lesions following perinatal hypoxic–ischaemic injury. Eur J Paediatr Neurol. 2012;16:688–96.
Krüger E, Kritzinger A, Pottas L. Oropharyngeal dysphagia in breastfeeding neonates with hypoxic-ischemic encephalopathy on therapeutic hypothermia. Breastfeed Med. 2019;14:718–23.
Gupta S, Bapuraj JR, Carlson G, Trumpower E, Dechert RE, Sarkar S. Predicting the need for home gavage or g-tube feeds in asphyxiated neonates treated with therapeutic hypothermia. J Perinatol J Calif Perinat Assoc. 2018;38:728–33.
Rohrer MJ, Natale AM. Effect of hypothermia on the coagulation cascade. Crit Care Med. 1992;20:1402–5.
Shankaran S, Pappas A, Laptook AR, McDonald SA, Ehrenkranz RA, Tyson JE, et al. Outcomes of safety and effectiveness in a multicenter randomized, controlled trial of whole-body hypothermia for neonatal hypoxic-ischemic encephalopathy. Pediatrics. 2008;122:e791–8.
Acknowledgements
We wish to thank the research coordinators and bedside nurses involved in the study, and we are indebted to the parents for their willingness to allow their children to participate in this study. We also wish to thank Tim Bahr, MD, with the University of Utah Department of Pediatrics and Yue Zhang and Zhining Ou with the University of Utah Study Design and Biostatistics Center for their statistical support. We would also like to thank our data safety monitoring committee, John Phillips, MD, Kristi Watterberg, MD, Pablo Sanchez, MD, and Lauren Jantzie, PhD.
Funding
This project was supported by the National Center for Research Resources and the National Center for Advancing Translational Science of the National Institute of Health through Grant Number UL1TR001449.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
DuPont, T.L., Baserga, M., Lowe, J. et al. Darbepoetin as a neuroprotective agent in mild neonatal encephalopathy: a randomized, placebo-controlled, feasibility trial. J Perinatol 41, 1339–1346 (2021). https://doi.org/10.1038/s41372-021-01081-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41372-021-01081-y
This article is cited by
-
Mild hypoxic-ischemic encephalopathy (HIE): timing and pattern of MRI brain injury
Pediatric Research (2022)