Abstract
Objective
To investigate seasonality and temporal trends in the incidence of NEC.
Study design
A retrospective cohort study from two tertiary NICUs in northern and central Connecticut involving 16,761 infants admitted over a 28-year period. Various perinatal and neonatal risk factors were evaluated by univariate, multivariate, and spectral density analyses.
Results
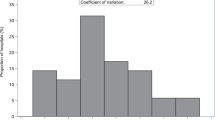
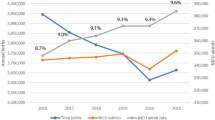
Incidence of NEC was unchanged over the 28 years of study. Gestational age, birth weight, and birth-months (birth in April/May) were independently associated with stage II or III NEC even after adjusting for confounding factors (p < 0.05). Yearly NEC incidence showed a multi-modal distribution with spectral density spikes approximately every 10 years.
Conclusion(s)
Temporal and seasonal factors may play a role in NEC with a peak incidence in infants born in April/May and periodicity spikes approximately every 10 years. These trends suggest non-random and possibly environmental factors influencing NEC.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Rossier A, Sarrut S, Delplanque J. [Ulcero-necrotic enterocolitis in premature infants]. La semaine des hopitaux: organe fonde par l’Association d’enseignement medical des hopitaux de Paris. 1959;35:1428–1436/P.
Rabl R. [Necrotizing enterocolitis in premature infants]. Beitrage zur pathologischen Anat und zur allgemeinen Pathologie. 1957;117:266–82.
Kelleher J, Mallick H, Soltau TD, Harmon CM, Dimmitt RA. Mortality and intestinal failure in surgical necrotizing enterocolitis. J Pediatr Surg. 2013;48:568–72.
Gordon PV, Swanson JR, MacQueen BC, Christensen RD. A critical question for NEC researchers: Can we create a consensus definition of NEC that facilitates research progress? Semin Perinatol. 2017;41:7–14.
Frost BL, Modi BP, Jaksic T, Caplan MS. New Medical and Surgical Insights Into Neonatal Necrotizing Enterocolitis: A Review. JAMA Pediatr. 2017;171:83–8.
Bell MJ, Shackelford P, Feigin RD, Ternberg JL, Brotherton T. Epidemiologic and bacteriologic evaluation of neonatal necrotizing enterocolitis. J Pediatr Surg. 1979;14:1–4.
Berdon WE, Grossman H, Baker DH, Mizrahi A, Barlow O, Blanc WA. Necrotizing enterocolitis in the premature infant. Radiology. 1964;83:879–87.
Seeman SM, Mehal JM, Haberling DL, Holman RC, Stoll BJ. Infant and maternal risk factors related to necrotizing enterocolitis-associated infant death in the United States. Acta Paediatr. 2016;105:e240–e246.
Martin FG, Saenz de Pipaon M, Perez Rodriguez J, Jimenez JQ. Risk factors for the development of necrotizing enterocolitis: a case-control study. J Neonatal-Perinat Med. 2013;6:311–8.
Derienzo C, Smith PB, Tanaka D, Bandarenko N, Campbell ML, Herman A, et al. Feeding practices and other risk factors for developing transfusion-associated necrotizing enterocolitis. Early Hum Dev. 2014;90:237–40.
Somaschini M, Breda-Klobus A, Pacati I. [Necrotizing enterocolitis (nec): risk factors and genetic susceptibility]. Minerva Pediatr. 2012;64:33–40.
Carter BM, Holditch-Davis D. Risk factors for necrotizing enterocolitis in preterm infants: how race, gender, and health status contribute. Adv Neonatal Care. 2008;8:285–90.
Hallstrom M, Koivisto AM, Janas M, Tammela O. Frequency of and risk factors for necrotizing enterocolitis in infants born before 33 weeks of gestation. Acta Paediatr. 2003;92:111–3.
Tapia-Rombo CA, Velasco-Lavin MR, Nieto-Caldelas A. [Risk factors of necrotizing enterocolitis]. Bol Med Hosp Infant Mex. 1993;50:650–4.
Zhong L, Hu T, Liu M. [Investigation on high risk factors of neonatal necrotizing enterocolitis]. Hua Xi Yi Ke Da Xue Xue Bao. 1991;22:311–3.
Milner ME, de la Monte SM, Moore GW, Hutchins GM. Risk factors for developing and dying from necrotizing enterocolitis. J Pediatr Gastroenterol Nutr. 1986;5:359–64.
Yu VY, Joseph R, Bajuk B, Orgill A, Astbury J. Perinatal risk factors for necrotizing enterocolitis. Arch Dis Child. 1984;59:430–4.
Wilson R, Kanto WP Jr., McCarthy BJ, Feldman RA. Age at onset of necrotizing enterocolitis. Risk factors in small infants. Am J Dis Child. 1982;136:814–6.
Kliegman RM, Hack M, Jones P, Fanaroff AA. Epidemiologic study of necrotizing enterocolitis among low-birth-weight infants. Absence of identifiable risk factors. J Pediatr. 1982;100:440–4.
Rodriguez Luis JC, Moya M, Domenech E, Mendez A. [Hypothermia, respiratory distress and umbilical catheterization as risk factors in necrotizing enterocolitis (author’s transl)]. Esp Pediatr. 1981;15:258–63.
Yu VY, Tudehope DI. Neonatal necrotizing enterocolitis: 2. Perinatal risk factors. Med J Aust. 1977;1:688–93.
Snyder CL, Hall M, Sharma V, St Peter SD. Seasonal variation in the incidence of necrotizing enterocolitis. Pediatr Surg Int. 2010;26:895–8.
Ahle M, Drott P, Andersson RE. Epidemiology and trends of necrotizing enterocolitis in Sweden: 1987-2009. Pediatrics. 2013;132:e443–51.
Meinzen-Derr J, Morrow AL, Hornung RW, Donovan EF, Dietrich KN, Succop PA. Epidemiology of necrotizing enterocolitis temporal clustering in two neonatology practices. J Pediatr. 2009;154:656–61.
Bell MJ, Ternberg JL, Feigin RD, Keating JP, Marshall R, Barton L, et al. Neonatal necrotizing enterocolitis. Therapeutic decisions based upon clinical staging. Ann Surg. 1978;187:1–7.
Gordon PV, Attridge JT. Understanding clinical literature relevant to spontaneous intestinal perforations. Am J Perinatol. 2009;26:309–16.
Attridge JT, Clark R, Walker MW, Gordon PV. New insights into spontaneous intestinal perforation using a national data set: (2) two populations of patients with perforations. J Perinatol. 2006;26:185–8.
Northway WH Jr, Rosan RC, Porter DY. Pulmonary disease following respirator therapy of hyaline-membrane disease. Bronchopulmonary dysplasia. N Engl J Med. 1967;276:357–68.
Papile LA, Burstein J, Burstein R, Koffler H. Incidence and evolution of subependymal and intraventricular hemorrhage: a study of infants with birth weights less than 1,500 gm. J Pediatr. 1978;92:529–34.
International Committee for the Classification of Retinopathy of P. The International Classification of Retinopathy of Prematurity revisited. Arch Ophthalmol. 2005;123:991–9.
W. Autocovariance. In: Statistics: Wikipedia. Wikipedia, The Free Encyclopedia; 2019. p. 979056055.
Virtanen P, Gommers R, Oliphant TE, Haberland M, Reddy T, Cournapeau D, et al. SciPy 1.0: fundamental algorithms for scientific computing in Python. Nature methods. 2020;17:261–72.
Cook SM, Glass RI, LeBaron CW, Ho MS. Global seasonality of rotavirus infections. Bull World Health Organ. 1990;68:171–7.
Ahle S, Badru F, Damle R, Osei H, Munoz-Abraham AS, Bajinting A, et al. Multicenter retrospective comparison of spontaneous intestinal perforation outcomes between primary peritoneal drain and primary laparotomy. J Pediatr Surg. 2019;55:1270–5.
Murphy T, Yang S, Tucker R, Collyer H, Kurkchubasche AG, Bender J. Necrotizing enterocolitis and spontaneous intestinal perforation: a spatiotemporal case cluster analysis. Pediatr Qual Saf. 2019;4:e127.
Dowdle WR. Influenza pandemic periodicity, virus recycling, and the art of risk assessment. Emerg Infect Dis. 2006;12:34–9.
Yee WH, Soraisham AS, Shah VS, Aziz K, Yoon W, Lee SK. Incidence and timing of presentation of necrotizing enterocolitis in preterm infants. Pediatrics. 2012;129:e298–304.
Worsham C, Woo J, Jena AB. Birth month and influenza vaccination in children. N. Engl J Med. 2020;383:184–5.
McCormack CJ, Emmens RW, Putnam TC. Evaluation of factors in high risk neonatal necrotizing enterocolitis. J Pediatr Surg. 1987;22:488–91.
Grosfeld JL, Chaet M, Molinari F, Engle W, Engum SA, West KW, et al. Increased risk of necrotizing enterocolitis in premature infants with patent ductus arteriosus treated with indomethacin. Ann Surg. 1996;224:350–5.
Luig M, Lui K, Nsw, Group AN. Epidemiology of necrotizing enterocolitis–Part II: Risks and susceptibility of premature infants during the surfactant era: a regional study. J Paediatr Child Health. 2005;41:174–9.
Gephart SM, McGrath JM, Effken JA, Halpern MD. Necrotizing enterocolitis risk: state of the science. Adv Neonatal Care. 2012;12:77–87.
Samuels N, van de Graaf RA, de Jonge RCJ, Reiss IKM, Vermeulen MJ. Risk factors for necrotizing enterocolitis in neonates: a systematic review of prognostic studies. BMC Pediatr. 2017;17:105.
Major CA, Lewis DF, Harding JA, Porto MA, Garite TJ. Tocolysis with indomethacin increases the incidence of necrotizing enterocolitis in the low-birth-weight neonate. Am J Obstet Gynecol. 1994;170:102–6.
Culhane JF, Goldenberg RL. Racial disparities in preterm birth. Semin Perinatol. 2011;35:234–9.
Jen HC, Graber JJ, Hill JL, Alaish SM, Voigt RW, Strauch ED. Surgical necrotizing enterocolitis and intraventricular hemorrhage in premature infants below 1000 g. J Pediatr Surg. 2006;41:1425–30.
Hall NJ, Hiorns M, Tighe H, Peters M, Khoo AK, Eaton S, et al. Is necrotizing enterocolitis associated with development or progression of intraventricular hemorrhage? Am J Perinatol. 2009;26:139–43.
Burns JC, Cayan DR, Tong G, Bainto EV, Turner CL, Shike H, et al. Seasonality and temporal clustering of Kawasaki syndrome. Epidemiology. 2005;16:220–5.
Torok TJ, Kilgore PE, Clarke MJ, Holman RC, Bresee JS, Glass RI. Visualizing geographic and temporal trends in rotavirus activity in the United States, 1991 to 1996. National Respiratory and Enteric Virus Surveillance System Collaborating Laboratories. Pediatr Infect Dis J. 1997;16:941–6.
Sharma R, Garrison RD, Tepas JJ 3rd, Mollitt DL, Pieper P, Hudak ML, et al. Rotavirus-associated necrotizing enterocolitis: an insight into a potentially preventable disease? J Pediatr Surg. 2004;39:453–7.
Coggins SA, Wynn JL, Weitkamp JH. Infectious causes of necrotizing enterocolitis. Clin Perinatol. 2015;42:133–54.
Lodha A, de Silva N, Petric M, Moore AM. Human torovirus: a new virus associated with neonatal necrotizing enterocolitis. Acta Paediatr. 2005;94:1085–8.
Jamieson FB, Wang EE, Bain C, Good J, Duckmanton L, Petric M. Human torovirus: a new nosocomial gastrointestinal pathogen. J Infect Dis. 1998;178:1263–9.
Birenbaum E, Handsher R, Kuint J, Dagan R, Raichman B, Mendelson E, et al. Echovirus type 22 outbreak associated with gastro-intestinal disease in a neonatal intensive care unit. Am J Perinatol. 1997;14:469–73.
Castro R. Echovirus 7 infection and necrotizing enterocolitis-like symptoms in a premature infant. J Perinatol. 2000;20:558–61.
Yolken RH, Franklin CC. Gastrointestinal adenovirus: an important cause of morbidity in patients with necrotizing enterocolitis and gastrointestinal surgery. Pediatr Infect Dis. 1985;4:42–7.
Turcios-Ruiz RM, Axelrod P, St John K, Bullitt E, Donahue J, Robinson N, et al. Outbreak of necrotizing enterocolitis caused by norovirus in a neonatal intensive care unit. J Pediatr. 2008;153:339–44.
Bagci S, Eis-Hubinger AM, Yassin AF, Simon A, Bartmann P, Franz AR, et al. Clinical characteristics of viral intestinal infection in preterm and term neonates. Eur J Clin Microbiol Infect Dis. 2010;29:1079–84.
Panesso-Gomez S, Shimamura M, Conces M, Talavera MM, Moallem M, Sanchez PJ, et al. Detection of Cytomegalovirus in Intestinal Tissue of Infants with Necrotizing Enterocolitis or Spontaneous Intestinal Perforation. J Pediatr. 2019;214:34–40.
Bagci S, Eis-Hubinger AM, Franz AR, Bierbaum G, Heep A, Schildgen O, et al. Detection of astrovirus in premature infants with necrotizing enterocolitis. Pediatr Infect Dis J. 2008;27:347–50.
Acknowledgements
The authors would like to acknowledge the support of the University of Connecticut, UConn Health, and Connecticut Children’s Medical Center in this project. Specifically, we would like to acknowledge the support of UConn-Office of Undergraduate Research that provided a student research grant for DJ.
Funding
Funding for this project was partially provided by a UConn Health Research Program grant for DJ through the Office of Undergraduate Research.
Author information
Authors and Affiliations
Contributions
DJ aggregated and organized the patient data, performed the statistical analysis, and prepared the manuscript. ZW performed the spectrum analysis and assisted in statistical analysis. SR supervised and mentored the contributions of ZW. NH conceptualized this project, designed the study, and supervised DJ in the data aggregation and analysis. He also mentored DJ in writing and editing the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Javidi, D., Wang, Z., Rajasekaran, S. et al. Temporal and seasonal variations in incidence of stage II and III NEC—a 28-year epidemiologic study from tertiary NICUs in Connecticut, USA. J Perinatol 41, 1100–1109 (2021). https://doi.org/10.1038/s41372-021-00961-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41372-021-00961-7
This article is cited by
-
A quality improvement initiative to reduce necrotizing enterocolitis in high-risk neonates
Journal of Perinatology (2023)
-
Machine learning-based risk factor analysis of necrotizing enterocolitis in very low birth weight infants
Scientific Reports (2022)
-
Spontaneous intestinal perforation (SIP) will soon become the most common form of surgical bowel disease in the extremely low birth weight (ELBW) infant
Journal of Perinatology (2022)