Abstract
Background:
Quantitative MRI techniques help recognize delayed brain development in fetuses with congenital heart disease (CHD). Ventriculomegaly became an early marker of brain dysmaturity.
Objective:
Evaluate longitudinally the cerebral ventricular and total brain volumes (TBV) in infants with CHD compared to normal neonates: testing the fetal brain dysmaturity and following its progression post operatively.
Study design:
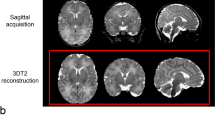
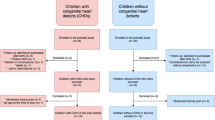
Fetal and post-operative MRIs were obtained on fetuses/neonates with CHD requiring invasive intervention within the first month after birth. Volumetric measurement was done with ITK-SNAP and analyzed post-hoc.
Results:
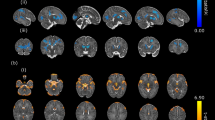
Ten cases were evaluated with a significant decrease in ventricular volumes from the fetal to the post-operative neonatal timepoint (p = 0.0297). Infants with HLHS had a significant increase postoperatively in their TBV (p = 0.0396).
Conclusions:
TBV increased post operatively inversely mirrored by the decrement of the ventricular volumes. This could be explained by the establishment an increase of brain blood flow after surgery.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Miller SP, McQuillen PS. Neurology of congenital heart disease: insight from brain imaging. Arch Dis Child-Fetal Neonatal Ed. 2007;92:F435–F437.
Hoffman JI, Kaplan S. The incidence of congenital heart disease. J Am Coll Cardiol. 2002;39:1890–1900.
Smyser CD, Neil JJ. Use of resting-state functional MRI to study brain development and injury in neonates. Semin Perinatol 2015;39:130–40.
Bellinger DC, Wypij D, duPlessis AJ, Rappaport, LA, Jonas, RA, Wernovsky, G. et al. Neurodevelopmental status at eight years in children with dextro-transposition of the great arteries: the Boston Circulatory Arrest Trial. J Thorac Cardiovasc Surg. 2003;126:1385–96.
Gaynor JW, Gerdes M, Zackai EH, et al. Apolipoprotein E genotype and neurodevelopmental sequelae of infant cardiac surgery. J Thorac Cardiovasc Surg. 2003;126:1736–45.
Von Rhein M, Buchmann A, Hagmann C, et al. Brain volumes predict neurodevelopment in adolescents after surgery for congenital heart disease. Brain 2014;137:268–76.
Rogers BT, Msall ME, Buck GM, et al. Neurodevelopmental outcome of infants with hypoplastic left heart syndrome. J Pediatr 1995;126:496–8.
Mebius MJ, Kooi EMW, Bilardo CM, Bos AF. Brain injury and neurodevelopmental outcome in congenital heart disease: a systematic review. Pediatrics. 2017;140:20164055.
Kelly CJ, Makropoulos A, Cordero-Grande L, et al. Impaired development of the cerebral cortex in infants with congenital heart disease is correlated to reduced cerebral oxygen delivery. Sci Rep. 2017;7:15088.
Kazan-Tannus JF, Dialani V, Chiang G, Feldman HA, Brown J, Levine D. Brain volumetry in fetuses referred for ventriculomegaly. AJR Am J Roentgenol. 2007;189:145.
Karl TR, Hall S, Ford G, et al. Arterial switch with full-flow cardiopulmonary bypass and limited circulatory arrest: neurodevelopmental outcome. J Thorac Cardiovasc Surg. 2004;127:213–22.
Limperopoulos C, Majnemer A, Shevell MI, et al. Predictors of developmental disabilities after open heart surgery in young children with congenital heart defects. J Pediatr 2002;141:51–58.
du Plessis AJ. Mechanisms of brain injury during infant cardiac surgery. Semin Pediatr Neurol. 1999;6:32–47.
Ransom J, Srivastava D. The genetics of cardiac birth defects. Semin Cell Dev Biol. 2007;18:132–9.
Clouchoux C, Guizard N, Evans AC, du Plessis AJ, Limperopoulos C. Normative fetal brain growth by quantitative in vivo magnetic resonance imaging. Am J Obstet Gynecol. 2012;206:173.e1–173.e8.
Lyons K, Cassady C, Jones J, et al. Current role of fetal magnetic resonance imaging in neurologic anomalies. Semin Ultrasound CT MR. 2015;36:298–309.
Licht DJ, Shera DM, Clancy RR, et al. Brain maturation is delayed in infants with complex congenital heart defects. J Thorac Cardiovasc Surg. 2009;137:529–36-537.
Ortinau C, Inder T, Lambeth J, Wallendorf M, Finucane K, Beca J. Congenital heart disease affects cerebral size but not brain growth. Pediatr Cardiol 2012;33:1138–46.
Rousseau F, Oubel E, Pontabry J, et al. BTK: an open-source toolkit for fetal brain MR image processing. Comput Methods Prog Biomed. 2013;109:65–73.
Masoller N, Sanz-Cortés M, Crispi F, et al. Severity of fetal brain abnormalities in congenital heart disease in relation to the main expected pattern of in utero brain blood supply. Fetal Diagn Ther. 2016;39:269–78.
Masoller N, Sanz-CortéS M, Crispi F, et al. Mid-gestation brain Doppler and head biometry in fetuses with congenital heart disease predict abnormal brain development at birth. Ultrasound Obstet Gynecol. 2016;47:65–73.
Khalil A, Bennet S, Thilaganathan B, Paladini D, Griffiths P, Carvalho JS. Prevalence of prenatal brain abnormalities in fetuses with congenital heart disease: a systematic review. Ultrasound Obstet Gynecol. 2016;48:296–307.
Clouchoux C, du Plessis AJ, Bouyssi-Kobar M, et al. Delayed cortical development in fetuses with complex congenital heart disease. Cereb Cortex 2013;23:2932–43.
Schellen C, Ernst S, Gruber GM, et al. Fetal MRI detects early alterations of brain development in Tetralogy of Fallot. Am J Obstet Gynecol. 2015;213:392.e1–7.
Limperopoulos C, Tworetzky W, McElhinney DB, et al. Brain volume and metabolism in fetuses with congenital heart disease: evaluation with quantitative magnetic resonance imaging and spectroscopy. Circulation 2010;121:26–33.
Sun L, Macgowan CK, Sled JG, et al. Reduced fetal cerebral oxygen consumption is associated with smaller brain size in fetuses with congenital heart disease. Circulation 2015;131:1313–23.
Ortinau C, Inder T, Lambeth J, Wallendorf M, Finucane K, Beca J. Congenital heart disease affects cerebral size but not brain growth. Pediatr Cardiol 2012;33:1138–46.
Kelly CJ, Makropoulos A, Cordero-Grande L, et al. Impaired development of the cerebral cortex in infants with congenital heart disease is correlated to reduced cerebral oxygen delivery. Sci Rep. 2017;7:15088.
Gaynor JW. The encephalopathy of congenital heart disease. J Thorac Cardiovasc Surg. 2014;148:1790–1.
Lynch JM, Buckley EM, Schwab PJ, et al. Time to surgery and preoperative cerebral hemodynamics predict postoperative white matter injury in neonates with hypoplastic left heart syndrome. J Thorac Cardiovasc Surg. 2014;148:2181–8.
Brossard-Racine M, du Plessis A, Vezina G, et al. Brain Injury in Neonates with Complex Congenital Heart Disease: What Is the Predictive Value of MRI in the Fetal Period? AJNR Am. J. Neuroradiol. 2016;37:1338–46.
Gholipour A, Estroff JA, Barnewolt CE, Connolly SA, Warfield SK. Fetal brain volumetry through MRI volumetric reconstruction and segmentation. Int J Comput Assist Radiol Surg. 2011;6:329–39.
Grossman R, Hoffman C, Mardor Y, Biegon A. Quantitative MRI measurements of human fetal brain development in utero. NeuroImage 2006;33:463–70.
Gong QY, Roberts N, Garden AS, Whitehouse GH. Fetal and fetal brain volume estimation in the third trimester of human pregnancy using gradient echo MR imaging. Magn Reson Imaging. 1998;16:235–40.
Scott JA, Habas PA, Kim K, et al. Growth trajectories of the human fetal brain tissues estimated from 3D reconstructed in utero MRI. Int J Dev Neurosci. 2011;29:529–36.
Acknowledgements
We would like to thank the dedicated teams of the USC Fetal Maternal Care Center, CHLA Radiology, and the intensive care units who take care of these sick infants and their families.
Funding
This paper is supported by the Children’s Heart Foundation, Southern California Clinical, and Translational Science Institute, the Pittsburgh Children’s Foundation, the National Institutes of Health, the Gerber Foundation, and CHLA Clinical Services; and also supported by The Children’s Heart Foundation and Children’s Hospital Los Angeles Clinical Trials Unit.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Hayek, C., Rajagopalan, V., Meouchy, J. et al. Ventricular and total brain volumes in infants with congenital heart disease: a longitudinal study. J Perinatol 40, 1383–1388 (2020). https://doi.org/10.1038/s41372-020-0711-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41372-020-0711-4