Abstract
Objective
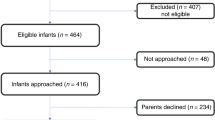
To assess whether in very preterm infants (1) body mass index (BMI) Z-score and weight-for-length (WtFL) Z-score at 1 year of age and (2) head growth from discharge to 1 year are associated with breastfeeding at discharge and the age of onset and type of complementary foods.
Study design
Observational cohort study.
Results
Infants started on only ready-made complementary (RMC) feedings at ≤26 weeks adjusted age had the highest adjusted BMI Z-score and WtFL Z-score at 1 year of age. Adjusted change in fronto-occipital circumference was highest in infants either discharged on breastmilk or receiving home-made complementary food with/without RMC (HMM) at ≤26 weeks adjusted age.
Conclusions
Infants started on RMC ≤26 weeks adjusted age had the highest BMI Z-score and WtFL Z-score at 1 year. Head growth from discharge to 1 year was highest in infants either discharged on breastmilk or receiving HMM at ≤26 weeks adjusted age.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Duncan AF, Heyne RJ, Morgan JS, Ahmad N, Rosenfeld CR. Elevated systolic blood pressure in preterm very-low-birth-weight infants ≤3 years of life. Pediatr Nephrol. 2011;26:1115–21.
Frankfurt JA, Duncan AF, Heyne RJ, Rosenfeld CR. Renal function and systolic blood pressure in very-low-birth-weight infants 1-3 years of age. Pediatr Nephrol. 2012;27:2285–91.
Duncan AF, Frankfurt JA, Heyne RJ, Rosenfeld CR. Biomarkers of adiposity are elevated in preterm very-low-birth-weight infants at 1, 2 and 3 Y of age. Pediatr Res. 2017;81:780–6.
Cooke RJ, Griffin I. Altered body composition in preterm infants at hospital discharge. Acta Paediatr. 2009;98:1269–73.
Stokes TA, Holston A, Olsen C, Choi Y, Curtis J, Higginson J, et al. Preterm infants of lower gestational age at birth have greater waist circumference-length ratio and ponderal index at term age than preterm infants of higher gestational ages. J Pediatr. 2012;161:735–41.
Johnson MJ, Wootton SA, Leaf AA, Jackson AA. Preterm birth and body composition at term equivalent age: a systematic review and meta-analysis. Pediatrics. 2012;130:e640–e649.
Wickland J, Heyne R, Brown LS, Turer CB, Rosenfeld CR. Preterm very-low-birthweight (PT-VLBW) infants continue to exhibit high systolic blood pressure (SBP) and altered renal function at preadolescence. In: Proceedings of the AAP 94th Perinatal & Developmental Medicine Symposium, Snow Mass: CO; 2019.
Pavageau L, Rosenfeld CR, Heyne RJ, Brown LS, Whitham J, Lair C, et al. Valid serial length measurements in preterm infants permit characterization of growth patterns. J Perinatol. 2018;38:1694–701.
Brion LP, Rosenfeld CR, Heyne R, Brown LS, Lair C, Burchfield P, et al. Adjustable feedings plus accurate serial length measurements decrease weight-length disproportion in very preterm infants. J Perinatol. 2019;39:1131–39.
Ferguson MC, O’Shea KJ, Hammer LD, Hertenstein DL, Schwartz NJ, Winch LE, et al. The impact of following solid food feeding guides on BMI among infants: a simulation study. Am J Prev Med. 2019. https://doi.org/10.1016/j.amepre.2019.04.011.
Agostini C, Buonocore G, Carnielli VP, De Curtis M, Darmaun D, Decsi T, et al. Enteral nutrient supply for preterm infants: commentary from the European Society of Paediatric Gastroenterology, Hepatology and Nutrition Committee on nutrition. J Pediatr Gastroenterol Nutr. 2010;50:85–91.
Lapillonne A, O’Connor L, Wang D, Rigo J. Nutritional recommendations for the late-preterm infant and the preterm infant after hospital discharge. J Pediatr. 2013;162:S90–100.
Arslanoglu S, Boquien CY, King C, Lamireau D, Tonetto P, Barnett D. Fortification of human milk for preterm infants: update and recommendations of the European Milk Bank Association (EMBA) Working Group on human milk fortification. Front Pediatr. 2019;7:76.
Kumar RK, Singhal A, Vaidya U, Banerjee S, Anwar F, Rao S. Optimizing nutrition in preterm low birth weight infants-consensus summary. Front Nutr. 2017;4:20.
Henderson G, Fahey T, McGuire W. Nutrient-enriched formula milk versus human breast milk for preterm infants following hospital discharge. Cochrane Database Syst Rev. 2007;4:CD004862.
Young L, Embleton ND, McCormick FM, McGuire W. Multinutrient fortification of human breast milk for preterm infants following hospital discharge. Cochrane Database Syst Rev. 2013;28:CD004866.
Vissers KM, Feskens EJM, van Goudoever JB, Janse AJ. The timing of initiating complementary feeding in preterm infants and its effect on overweight: a systematic review. Ann Nutr Metab. 2018;72:307–15.
Teller IC, Embleton ND, Griffin IJ, van Elburg RM. Post-discharge formula feeding in preterm infants: a systematic review mapping evidence about the role of macronutrient enrichment. Clin Nutr. 2016;35:791–801.
Embleton NE, Pang N, Cooke RJ. Postnatal malnutrition and growth retardation: an inevitable consequence of current recommendations in preterm infants? Pediatrics. 2001;107:270–3.
Olsen IE, Richardson DK, Schmid CH, Ausman LM, Dwyer JT. Intersite differences in weight growth velocity of extremely premature infants. Pediatrics. 2002;110:1125–32.
Rozé JC, Darmaun D, Boquien CY, Flamant C, Picaud JC, Savagner C, et al. The apparent breastfeeding paradox in very preterm infants: relationship between breast feeding, early weight gain and neurodevelopment based on results from two cohorts, EPIPAGE and LIFT. BMJ Open. 2012;2:e000834.
Morgan JB, Lucas A, Fewtrell MS. Does weaning influence growth and health up to 18 months? Arch Dis Child. 2004;89:728–33.
Gupta S, Agarwal R, Aggarwal KC, Chellani H, Duggal A, Arya S, et al. Complementary feeding at 4 versus 6 months of age for preterm infants born at less than 34 weeks of gestation: a randomised, open-label, multicentre trial. Lancet Glob Health. 2017;5:e501–e511.
Marriott LD, Foote KD, Bishop JA, Kimber AC, Morgan JB. Weaning preterm infants: a randomised controlled trial. Arch Dis Child Fetal Neonatal Ed. 2003;88:F302–F307.
Broyles RS, Tyson JE, Heyne ET, Heyne RJ, Hickman JF, et al. Comprehensive follow-up care and life-threatening illnesses among high-risk infants: a randomized controlled trial. JAMA. 2000;284:2070–6.
Olsen IE, Lawson ML, Ferguson AN, Cantrell R, Grabich SC, Zemel BS, et al. BMI curves for preterm infants. Pediatrics. 2015;135:e572–e581.
Olsen IE, Groveman SA, Lawson ML, Clark RH, Zemel BS. New intrauterine growth curves based on United States data. Pediatrics. 2010;125:e214–e224.
WHO Multicentre Growth Reference Study Group. WHO Child Growth Standards based on length/height, weight and age. Acta Paediatr. 2006;450:76.
WHO Multicentre Growth Reference Study Group. WHO child growth standards: length/height-for-age, weight-for-age, weight-for-length, weight-for-height and body mass index-for-age: methods and development. Geneva: World Health Organization; 2006. https://www.who.int/childgrowth/standards/Technical_report.pdf. Accessed 12 Feb 2018.
WHO Multicentre Growth Reference Study Group. WHO child growth standards: head circumference-for-age, arm circumference-for-age, triceps skinfold-for-age and subscapular skinfold-for-age: methods and development. Geneva: World Health Organization; 2007.
Emmett PM. Dietary patterns during complementary feeding and later outcomes. Nestlé Nutr Inst Workshop Ser. 2016;85:145–54.
de Onis M, Onyango A, Borghi E, Siyam A, Blössner M, Lutter C, WHO Multicentre Growth Reference Study Group. Worldwide implementation of the WHO Child Growth Standards. Public Health Nutr. 2012;15:1603–10.
Aris IM, Rifas-Shiman SL, Li LJ, Yang S, Belfort MB, Thompson J, et al. Association of weight for length vs body mass index during the first 2 years of life with cardiometabolic risk in early adolescence. JAMA Netw Open. 2018;1:e182460.
Furlong KR, Anderson LN, Kang H, Lebovic G, Parkin PC, Maguire JL, et al. BMI-for-age and weight-for-length in children 0 to 2 years. Pediatrics. 2016;138:e20153809.
Olsen IE, Harris CL, Lawson ML, Berseth Cl. Higher protein intake improves length, not weight, z scores in preterm infants. JPGN. 2014;58:409–16.
Allen LH. Nutritional influences on linear growth: a general review. Eur J Clin Nutr. 1994;48:S75–S89.
Roberts JL, Stein AD. The impact of nutritional interventions beyond the first 2 years of life on linear growth: a systematic review and meta-analysis. Am Soc Nutr Adv Nutr. 2017;8:323–836.
Rodriguez J, Affuso O, Azuero A, Downs CA, Turner-Henson A, Rice M. Infant feeding practices and weight gain in toddlers born very preterm: a pilot study. J Pediatr Nursing. 2018;43:29–35.
World Health Organization. Commercial foods for infants and young children in the WHO/European Region. A study of the availability, composition and marketing of baby foods in four European countries. http://www.euro.who.int/en/health-topics/disease-prevention/nutrition/publications/2019/commercial-foods-for-infants-and-young-children-in-the-who-european-region-2019, Accessed 3 Aug 2019.
Breij LM, Kerkhof GF, Hokken-Koelega ACS. Risk for nonalcoholic fatty liver diseasein young adults born preterm. Horm Res Paediatr. 2015;84:199–205.
Franz AR, Pohlandt F, Bode H, Mihatsch WA, Sander S, Kron M, et al. Intrauterine, early neonatal, and postdischarge growth and neurodevelopmental outcome at 5.4 years in extremely preterm infants after intensive neonatal nutritional support. Pediatrics. 2009;123:e101–e109.
Neubauer V, Fuchs T, Griesmaier E, Kager K, Pupp-Peglow U, Kiechl-Kohlendorfer U. Poor postdischarge head growth is related to a 10% lower intelligence quotient in very preterm infants at the chronological age of five years. Acta Paediatr. 2016;105:501–7.
Neubauer V, Griesmaier E, Pehböck-Walser N, Pupp-Peglow U, Kiechl-Kohlendorfer U. Poor postnatal head growth in very preterm infants is associated with impaired neurodevelopment outcome. Acta Paediatr. 2013;102:883–8.
Acknowledgements
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request. Susan Chacko, RN, and Maria DeLeon, RN, were research coordinators for part of this study. Chen Du, RD, Elizabeth Brammer, RD, Audrey Edwards, RD, and Theresa Jacob, RD, dietitians at Parkland Hospital, obtained anthropometric measurements for this study and participated in patient recruitment, assessments of growth and laboratory results and recommendations for nutritional interventions. Elen Petrosyan, RD, dietitian at Parkland Hospital, helped in organizing the logistics of patient recruitment, measurements, and research planning. Sandra Gosser, NNP, helped with education of nurse practitioners and coordination of care with the dietitians and the physicians. Timothy Brannon, MD, helped with changes on EPIC, specifically with implementation of parameters to define AGA at birth. Rebecca Thomas, RN and Catherine Vanbeek, RN, NP, helped with changes in EPIC ordering and education of nurses. Erin McDougald, PNP, Anna Puentez, PNP, Jillian Waterbury, PNP, Linda Madden, PNP, Sally Adams, PNP, Renea Powell, NNP, Jerithea Tidwell, PNP, Karen Malouf, RD, Shannon Despino, RD, Carly Brenner, RD, Marianna Skertchy, RD, Lizette Torres, RN, and Alicia Guzman helped with collection of follow-up data. Anne-Marie Rosenfeld, RN, helped with analysis and interpretation of the data. Some of the patients included in the QI described in the current paper were enrolled in a blinded randomized trial: Clinicaltrials.gov NCT02372136. The study sponsors had no role in design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review and approval of the paper; and decision to submit the paper for publication.
Funding
This study was funded by the George L. MacGregor Professorship (CR Rosenfeld) and Children’s Medical Center Clinical Advisory Committee (CCRAC)—Senior Investigator Research Award—New Direction (LP Brion).
Author information
Authors and Affiliations
Contributions
LPB wrote the first draft of the paper. He conceptualized and designed the study. He extracted data from medical records at the Thrive clinic, participated in the interpretation of the data, conducted statistical analyses, critically reviewed the revisions, and approved the final paper as submitted. CRR and RH conceptualized and designed the study. They participated in the interpretation of the data, critically reviewed the revisions, and approved the final paper as submitted. LSB conceptualized and designed the study. He conducted statistical analyses, participated in the interpretation of the data, critically reviewed the revisions, and approved the final paper as submitted. CSL conceptualized and designed nutritional guidelines for the NICU and discharge, anthropometric data, and data extraction from EPIC. She obtained anthropometric measurements for the QI and participated in assessments of growth and laboratory results and recommendations for nutritional interventions. She participated in the interpretation of the data, critically reviewed the revisions, and approved the final paper as submitted. ELD conceptualized and designed nutritional guidelines at and after discharge. ELD and EH standardized the interviews and data entry into outpatient electronic medical records, critically reviewed the revisions, and approved the final paper as submitted. PJB collected and entered the data into the databases. She participated in the interpretation of the data, critically reviewed the paper, and approved the final paper as submitted. MC was research coordinator for a large part of this study. She measured patients in the NICU and at follow-up. She extracted data from medical records into databases, critically reviewed the paper and approved the final paper as submitted.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This study was approved by the IRB of the University of Texas Southwestern Medical Center, Parkland Hospital and Health Systems and Children’s Medical Center.
Informed consent
Informed consent was waived for all data included in this study.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Preliminary results were submitted as abstract for presentation at PAS, Philadelphia, PA, May 2–5, 2020: Brion LP, Rosenfeld CR, Heyne R, Brown LS, Lair C, Heyne E, Dohoney E, Burchfield PJ, Caraig M. Association between age of initiation and type of complementary foods with body mass index (BMI) at 1 year of age in appropriate for gestational age (GA) 23–28 week-GA infants.
Supplementary information
Rights and permissions
About this article
Cite this article
Brion, L.P., Rosenfeld, C.R., Heyne, R. et al. Association of age of initiation and type of complementary foods with body mass index and weight-for-length at 12 months of age in preterm infants. J Perinatol 40, 1394–1404 (2020). https://doi.org/10.1038/s41372-020-0637-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41372-020-0637-x
This article is cited by
-
Follow-up of a randomized trial optimizing neonatal nutrition in preterm very low birthweight infants: growth, serum adipokines, renal function and blood pressure
Journal of Perinatology (2024)
-
Double-blinded randomized controlled trial of optimizing nutrition in preterm very low birth weight infants: Bayley scores at 18–38 months of age
Journal of Perinatology (2023)
-
Complementary feeding in preterm infants: a position paper by Italian neonatal, paediatric and paediatric gastroenterology joint societies
Italian Journal of Pediatrics (2022)
-
Quality improvement project designed to reduce disproportionate growth in extremely low gestational age neonates: cognitive neurodevelopmental outcome at 18–41 months
Journal of Perinatology (2021)
-
Zinc deficiency limiting head growth to discharge in extremely low gestational age infants with insufficient linear growth: a cohort study
Journal of Perinatology (2020)