Abstract
Objective
Examine the effect of off-label surfactant on mortality and morbidity in more mature and larger premature infants diagnosed with respiratory distress syndrome (RDS).
Study design
Cohort study of premature infants born at 30–36 weeks, birth weight > 2 kg, and a diagnosis of RDS. We compared the odds of mortality and morbidity between infants who were exposed vs unexposed to surfactant. We used a treatment effects model to balance covariates between groups.
Results
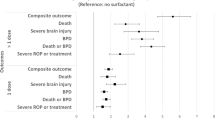
Of 54,964 included infants, 25,278 (46%) were exposed to surfactant. The frequency of mortality and morbidities were higher in the exposed group in unadjusted analyses. Following adjustment with a doubly robust treatment effects model, we found no significant treatment effect of surfactant on mortality or morbidity.
Conclusion
Surfactant exposure is not associated with reduced or increased mortality or morbidity in more mature premature infants with RDS.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Stoll BJ, Hansen NI, Bell EF, Shankaran S, Laptook AR, Walsh MC, et al. Neonatal outcomes of extremely preterm infants from the NICHD Neonatal Research Network. Pediatrics. 2010;126:443–56.
Hibbard JU, Wilkins I, Sun L, Gregory K, Haberman S, Hoffman M, et al. Respiratory morbidity in late preterm births. JAMA. 2010;304:419–25.
Polin RA, Carlo WA. Surfactant replacement therapy for preterm and term neonates with respiratory distress. Pediatrics. 2014;133:156–63.
Jobe AH. The new bronchopulmonary dysplasia. Curr Opin Pediatr. 2011;23:167–72.
AbbVie, Inc. Survanta [package label] AbbVie Inc, North Chicago, IL; 2013.
ONY, Inc. Infasurf [package label] ONY, Inc, Amherst, NY; 2011.
Chiesi Farmaceutici. Curosurf [package label] Chiesi Farmaceutici, Parma, Italy; 2014.
Koivisto M, Marttila R, Kurkinen-Raty M, Saarela T, Pokela ML, Jouppila P, et al. Changing incidence and outcome of infants with respiratory distress syndrome in the 1990s: a population-based survey. Acta Paediatr. 2004;93:177–84.
Bhat R, Dziedzic K, Bhutani VK, Vidyasagar D. Effect of single dose surfactant on pulmonary function. Crit Care Med. 1990;18:590–5.
Couser RJ, Ferrara TB, Ebert J, Hoekstra RE, Fangman JJ. Effects of exogenous surfactant therapy on dynamic compliance during mechanical breathing in preterm infants with hyaline membrane disease. J Pediatr. 1990;116:119–24.
Gitlin JD, Soll RF, Parad RB, Horbar JD, Feldman HA, Lucey JF, et al. Randomized controlled trial of exogenous surfactant for the treatment of hyaline membrane disease. Pediatrics. 1987;79:31–7.
Soll RF. Prophylactic natural surfactant extract for preventing morbidity and mortality in preterm infants. Cochrane database Syst Rev. 2000;2:Cd000511.
Suresh GK, Soll RF. Overview of surfactant replacement trials. J Perinatol. 2005;25 Suppl 2 :S40–4.
Taylor G, Jackson W, Hornik CP, Koss A, Mantena S, Homsley K, et al. Surfactant administration in preterm infants: drug development opportunities. J Pediatr. 2019;208:163–8.
Spitzer AR, Ellsbury DL, Handler D, Clark RH. The Pediatrix BabySteps Data Warehouse and the Pediatrix QualitySteps improvement project system–tools for “meaningful use” in continuous quality improvement. Clin Perinatol. 2010;37:49–70.
Trembath A, Hornik CP, Clark R, Smith PB, Daniels J, Laughon M. Comparative effectiveness of surfactant preparations in premature infants. J Pediatr. 2013;163:955–60.e1.
Soll RF, Blanco F. Natural surfactant extract versus synthetic surfactant for neonatal respiratory distress syndrome. Cochrane Database Syst Rev. 2001;2:Cd000144.
Yost CC, Soll RF. Early versus delayed selective surfactant treatment for neonatal respiratory distress syndrome. Cochrane Database Syst Rev. 2000;2:Cd001456.
Soll R, Ozek E. Multiple versus single doses of exogenous surfactant for the prevention or treatment of neonatal respiratory distress syndrome. Cochrane Database Syst Rev. 2009;1:Cd000141.
Abdel-Latif ME, Osborn DA. Nebulised surfactant in preterm infants with or at risk of respiratory distress syndrome. Cochrane Database Syst Rev. 2012;10:Cd008310.
Minocchieri S, Berry CA, Pillow JJ. Nebulised surfactant to reduce severity of respiratory distress: a blinded, parallel, randomised controlled trial. Arch Dis Child Fetal Neonatal Ed. 2019;104:F313–9.
Sood BG, Cortez J, Kolli M, Sharma A, Delaney-Black V, Chen X. Aerosolized surfactant in neonatal respiratory distress syndrome: phase I study. Early Hum Dev. 2019;134:19–25.
Ballard RA, Keller RL, Black DM, Ballard PL, Merrill JD, Eichenwald EC, et al. Randomized trial of late surfactant treatment in ventilated preterm infants receiving inhaled nitric oxide. J Pediatr. 2016;168:23–9.e4.
Laughon M, Bose C, Moya F, Aschner J, Donn SM, Morabito C, et al. A pilot randomized, controlled trial of later treatment with a peptide-containing, synthetic surfactant for the prevention of bronchopulmonary dysplasia. Pediatrics. 2009;123:89–96.
Dani C, Mosca F, Vento G, Tagliabue P, Picone S, Lista G, et al. Effects of surfactant treatment in late preterm infants with respiratory distress syndrome. J Matern Fetal Neonatal Med. 2018;31:1259–66.
Surmeli-Onay O, Korkmaz A, Yigit S, Yurdakok M. Surfactant therapy in late preterm infants: respiratory distress syndrome and beyond. Turk J Pediatr. 2012;54:239–46.
Wang H, Gao X, Liu C, Yan C, Lin X, Dong Y, et al. Surfactant reduced the mortality of neonates with birth weight 1500g and hypoxemic respiratory failure: a survey from an emerging NICU network. J Perinatol. 2017;37:645–51.
Saccone G, Berghella V. Antenatal corticosteroids for maturity of term or near term fetuses: systematic review and meta-analysis of randomized controlled trials. BMJ. 2016;355:i5044.
Wang C, Guo L, Chi C, Wang X, Guo L, Wang W, et al. Mechanical ventilation modes for respiratory distress syndrome in infants: a systematic review and network meta-analysis. Crit Care. 2015;19:108.
Sweet DG, Carnielli V, Greisen G, Hallman M, Ozek E, Plavka R, et al. European Consensus Guidelines on the Management of Respiratory Distress Syndrome—2016 Update. Neonatology. 2017;111:107–25.
Schmolzer GM, Kumar M, Pichler G, Aziz K, O'Reilly M, Cheung PY. Non-invasive versus invasive respiratory support in preterm infants at birth: systematic review and meta-analysis. BMJ. 2013;347:f5980.
Kamath BD, Macguire ER, McClure EM, Goldenberg RL, Jobe AH. Neonatal mortality from respiratory distress syndrome: lessons for low-resource countries. Pediatrics. 2011;127:1139–46.
Konishi M, Fujiwara T, Naito T, Takeuchi Y, Ogawa Y, Inukai K, et al. Surfactant replacement therapy in neonatal respiratory distress syndrome. A multi-centre, randomized clinical trial: comparison of high-versus low-dose of surfactant TA. Eur J Pediatr. 1988;147:20–5.
Davis DJ, Barrington KJ, Canadian Pediatric Society, Fetus and Newborn Committee. Recommendations for neonatal surfactant therapy. Paediatr Child Health. 2005;10:109–16.
Author information
Authors and Affiliations
Contributions
WJ, MML, DKB, and CPH conceived and designed the analysis. WJ and DKB performed the analysis and wrote the first draft of the manuscript. CPH, MML, GT, NAB, KZ, RC, and RGG reviewed and made significant edits to the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Jackson, W., Taylor, G., Bamat, N.A. et al. Outcomes associated with surfactant in more mature and larger premature infants with respiratory distress syndrome. J Perinatol 40, 1171–1177 (2020). https://doi.org/10.1038/s41372-020-0625-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41372-020-0625-1