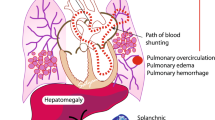
Abstract
Optimal management of patent ductus arteriosus (PDA) in extremely preterm infants remains controversial. There is paucity of evidence on the benefits of PDA treatment in reducing mortality and morbidities in extremely preterm infants. Failure of randomized clinical trials to demonstrate beneficial effects of PDA treatment on outcomes has often been attributed to open treatment of control subjects. This perspective examines the PDA treatment trials to date, with specific focus on rates of and ages of subjects at open rescue treatment. Although these trials demonstrate that ductal closure is significantly increased with treatment, that does not translate to a significant decrease in major morbidities or mortality in premature infants, even when trials with high rates of rescue treatment of controls are excluded. Trials in which enrollment occurred after 7 days of age include insufficient numbers of subjects to evaluate this relationship.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Semberova J, Sirc J, Miletin J, Kucera J, Berka I, Sebkova S, et al. Spontaneous closure of patent ductus arteriosus in infants ≤1500 g. Pediatrics. 2017;140:e20164258.
Benitz WE. Treatment of persistent patent ductus arteriosus in preterm infants: time to accept the null hypothesis? J Perinatol. 2010;30:241–52.
Clyman RI, Couto J, Murphy GM. Patent ductus arteriosus: are current neonatal treatment options better or worse than no treatment at all? Semin Perinatol. 2012;36:123–9.
Sankar MN, Bhombal S, Benitz WE. PDA: to treat or not to treat. Congenit Heart Dis. 2019;14:46–51.
Benitz WE, Bhombal S. The use of non-steroidal anti-inflammatory drugs for patent ductus arteriosus closure in preterm infants. Semin Fetal Neonatal Med. 2017;22:302–7.
Liebowitz M, Kaempf J, Erdeve O, Bulbul A, Hakansson S, Lindqvist J, et al. Comparative effectiveness of drugs used to constrict the patent ductus arteriosus: a secondary analysis of the PDA-TOLERATE trial (NCT01958320). J Perinatol. 2019;39:599–607.
Vali P, Lakshminrusimha S, Pelech A, Underwood M, Ing F. Patent ductus arteriosus in preterm infants: is early transcatheter closure a paradigm shift? J Perinatol. 2019;39:1449–61.
Evans N. Preterm patent ductus arteriosus: a continuing conundrum for the neonatologist? Semin Fetal Neonatal Med. 2015;20:272–7.
Clyman RI. Recommendations for the postnatal use of indomethacin: an analysis of four separate treatment strategies. J Pediatr. 1996;128:601–7.
Cooke L, Steer P, Woodgate P. Indomethacin for asymptomatic patent ductus arteriosus in preterm infants. Cochrane Database Syst Rev. 2003;2:CD003745.
Fowlie PW, Davis PG, McGuire W. Prophylactic intravenous indomethacin for preventing mortality and morbidity in preterm infants. Cochrane Database Syst Rev. 2010;7:CD000174.
Mitra S, Florez ID, Tamayo ME, Mbuagbaw L, Vanniyasingam T, Veroniki AA, et al. Association of placebo, indomethacin, ibuprofen, and acetaminophen with closure of hemodynamically significant patent ductus arteriosus in preterm infants: a systematic review and meta-analysis. JAMA. 2018;319:1221–38.
Ohlsson A, Walia R, Shah SS. Ibuprofen for the treatment of patent ductus arteriosus in preterm or low birth weight (or both) infants. Cochrane Database Syst Rev. 2018;9:CD003481.
Ohlsson A, Shah SS. Ibuprofen for the prevention of patent ductus arteriosus in preterm and/or low birth weight infants. Cochrane Database Syst Rev. 2019;6:CD004213.
Ohlsson A, Shah PS. Paracetamol (acetaminophen) for patent ductus arteriosus in preterm or low birth weight infants. Cochrane Database Syst Rev. 2020;1:CD010061.
Asbagh PA, Zarkesh MR, Nili F, Sadat NS, Naeem AT. Prophylactic treatment with oral paracetamol for patent ductus arteriosus in preterm infants: a randomized clinical trial. Tehran Univ Med J. 2015;73:86–92.
Bagnoli F, Rossetti A, Messina G, Mori A, Casucci M, Tomasini B. Treatment of patent ductus arteriosus (PDA) using ibuprofen: renal side-effects in VLBW and ELBW newborns. J Matern Fetal Neonatal Med. 2013;26:423–9.
Ding YJ, Han B, Yang B, Zhu M. NT-proBNP plays an important role in the effect of ibuprofen on preterm infants with patent ductus arteriosus. Eur Rev Med Pharmacol Sci. 2014;18:2596–8.
Neu J, Ariagno RL, Johnson JD, Pitlick PT, Cohen RS, Beets CL, et al. A double blind study of the effects of oral indomethacin in preterm infants with patent ductus arteriosus who failed medical management. Pediatr Pharmacol. 1981;1:245–9.
Cotton RB, Stahlman MT, Bender HW, Graham TP, Catterton WZ, Kovar I. Randomized trial of early closure of symptomatic patent ductus arteriosus in small preterm infants. J Pediatr. 1978;93:647–51.
Osborn DA, Evans N, Kluckow M. Effect of early targeted indomethacin on the ductus arteriosus and blood flow to the upper body and brain in the preterm infant. Arch Dis Child Fetal Neonatal Ed. 2003;88:F477–82.
Gersony WM, Peckham GJ, Ellison RC, Miettinen OS, Nadas AS. Effects of indomethacin in premature infants with patent ductus arteriosus: results of a national collaborative study. J Pediatr. 1983;102:895–906.
Deeks JJ, Higgins JPT, on behalf of the Statistical Methods Group of The Cochrane Collaboration. Statistical algorithms in review manager 5. https://training.cochrane.org/handbook/statistical-methods-revman5 (2010). Accessed 24 August 2020.
Yanagi RM, Wilson A, Newfeld EA, Aziz KU, Hunt CE. Indomethacin treatment for symptomatic patent ductus arteriosus: a double-blind control study. Pediatrics. 1981;67:647–52.
Mahony L, Carnero V, Brett C, Heymann MA, Clyman RI. Prophylactic indomethacin therapy for patent ductus arteriosus in very-low-birth-weight infants. N Engl J Med. 1982;306:506–10.
Nair PAK, Pai MG, Gazal HA, Da Costa DE, Al Khusaiby SM. Indomethacin prophylaxis for intraventricular hemorrhage in very low birth weight babies. Indian Pediatr. 2004;41:551–8.
Sosenko IR, Fajardo MF, Claure N, Bancalari E. Timing of patent ductus arteriosus treatment and respiratory outcome in premature infants: a double-blind randomized controlled trial. J Pediatr. 2012;160:929–35.
Clyman RI, Liebowitz M, Kaempf J, Erdeve O, Bulbul A, Hakansson S, et al. PDA-TOLERATE Trial: an exploratory randomized controlled trial of treatment of moderate-to-large patent ductus arteriosus at 1 week of age. J Pediatr. 2019;205:41–8.
Sung SI, Lee MH, Ahn SY, Chang YS, Park WS. Effect of nonintervention vs oral ibuprofen in patent ductus arteriosus in preterm infants: a randomized clinical trial. JAMA Pediatr. 2020. https://jamanetwork.com/journals/jamapediatrics/fullarticle/2766727.
Walsh MC, Wilson-Costello D, Zadell A, Newman N, Fanaroff A. Safety, reliability, and validity of a physiologic definition of bronchopulmonary dysplasia. J Perinatol. 2003;23:451–6.
Bell MJ, Ternberg JL, Feigin RD, Keating JP, Marshall R, Barton L, et al. Neonatal necrotizing enterocolitis. Therapeutic decisions based upon clinical staging. Ann Surg. 1978;187:1–7.
Ryan R, Hill S. How to GRADE the quality of the evidence [cited 2020 July 24]; Version 3.0. 2016. http://cccrg.cochrane.org/author-resources.
Levitsky S, Fisher E, Vidyasagar D, Hastreiter AR, Bennett E, Raju TN, et al. Interruption of patent ductus arteriosus in premature infants with respiratory distress syndrome. Ann Thorac Surg. 1976;22:131–7.
Cotton RB, Hickey DE, Graham TP, Stahlman MT. Effect of early indomethacin on ventilatory status of preterm infants with symptomatic patent ductus arteriosus [abstract]. Pediatr Res. 1980;14:442.
Nestrud RM, Hill DE, Arrington RW, Beard AG, Dungan WT, Lau PY, et al. Indomethacin treatment in patent ductus arteriosus. A double-blind study utilizing indomethacin plasma levels. Dev Pharmacol Ther. 1980;1:125–36.
Valaes T, Moylan FMB, Cohn H, Chung K, Nagpaul K, Chrenoff HI, et al. Incidence and significance of PDA in preterm infants (PTI) and controlled trial of indomethacin. Pediatr Res. 1980;14:452.
Merritt TA, Harris JP, Roghmann K, Wood B, Campanella V, Alexson C, et al. Early closure of the patent ductus arteriosus in very low-birth-weight infants: a controlled trial. J Pediatr. 1981;99:281–6.
Yeh TF, Luken JA, Thalji A, Raval D, Carr I, Pildes RS. Intravenous indomethacin therapy in premature infants with persistent ductus arteriosus-a double-blind controlled study. J Pediatr. 1981;98:137–45.
Mullett MD, Croghan TW, Myerberg DZ, Krall JM, Neal WA. Indomethacin for closure of patent ductus arteriosus in prematures. Clin Pediatr. 1982;21:217–20.
Yeh TF, Goldbarg HR, Henek T, Thalji A, Pildes RS. Intravenous indomethacin therapy in premature infants with patent ductus arteriosus. Causes of death and one-year follow-up. Am J Dis Child. 1982;136:803–7.
Kaapa P, Lanning P, Koivisto M. Early closure of patent ductus arteriosus with indomethacin in preterm infants with idiopathic respiratory distress syndrome. Acta Paediatr Scand. 1983;72:179–84.
Monset-Couchard M, Dias-Mancano D, Murat I, Relier JP. Controlled trial of intravenous lyophilized indomethacin in the treatment of persistent ductus arteriosus in premature infants. Pediatrie. 1983;38:365–77.
Rudd P, Montanez P, Hallidie-Smith K, Silverman M. Indomethacin treatment for patent ductus arteriosus in very low birthweight infants: double blind trial. Arch Dis Child. 1983;58:267–70.
Mahony L, Caldwell RL, Girod DA, Hurwitz RA, Jansen RD, Lemons JA, et al. Indomethacin therapy on the first day of life in infants with very low birth weight. J Pediatr. 1985;106:801–5.
Ment LR, Duncan CC, Ehrenkranz RA, Kleinman CS, Pitt BR, Taylor KJ, et al. Randomized indomethacin trial for prevention of intraventricular hemorrhage in very low birth weight infants. J Pediatr. 1985;107:937–43.
Puckett CG, Cox MA, Haskins KS, Fisher DJ. Prophylactic indomethacin for the prevention of patent ductus arteriosus [abstract]. Pediatr Res. 1985;19:358.
Benson JW, Drayton MR, Hayward C, Murphy JF, Osborne JP, Rennie JM, et al. Multicentre trial of ethamsylate for prevention of periventricular haemorrhage in very low birthweight infants. Lancet. 1986;2:1297–300.
Rennie JM, Doyle J, Cooke RW. Early administration of indomethacin to preterm infants. Arch Dis Child. 1986;61:233–8.
Gutierrez NG, Lapasset M. Prophylactic indomethacin and the incidence of patent ductus arteriosus in preterm neonates. In: Proceedings of the 3rd Argentinian Congress of Perinatology. 1987, 62: as cited in Fowlie PW, Davis PG, McGuire W: Prophylactic intravenous indomethacin for preventing mortality and morbidity in preterm infants. Cochrane Database Syst Rev 7:CD000174, 002010, p. 000132.
Hammerman C, Strates E, Komar K, Bui K. Failure of prophylactic indomethacin to improve the outcome of the very low birth weight infant. Dev Pharmacol Ther. 1987;10:393–404.
Krueger E, Mellander M, Bratton D, Cotton R. Prevention of symptomatic patent ductus arteriosus with a single dose of indomethacin. J Pediatr. 1987;111:749–54.
Vincer M, Allen A, Evans J, Nwaesei C, Stinson D, Rees E, et al. Early intravenous indomethacin prolongs respiratory support in very low birth weight infants. Acta Paediatr Scand. 1987;76:894–7.
Weesner KM, Dillard RG, Boyle RJ, Block SM. Prophylactic treatment of asymptomatic patent ductus arteriosus in premature infants with respiratory distress syndrome. South Med J. 1987;80:706–8.
Bandstra ES, Montalvo BM, Goldberg RN, Pacheco I, Ferrer PL, Flynn J, et al. Prophylactic indomethacin for prevention of intraventricular hemorrhage in premature infants. Pediatrics. 1988;82:533–42.
Hanigan WC, Kennedy G, Roemisch F, Anderson R, Cusack T, Powers W. Administration of indomethacin for the prevention of periventricular-intraventricular hemorrhage in high-risk neonates. J Pediatr. 1988;112:941–7.
Ment LR, Duncan CC, Ehrenkranz RA, Kleinman CS, Taylor KJ, Scott DT, et al. Randomized low-dose indomethacin trial for prevention of intraventricular hemorrhage in very low birth weight neonates. J Pediatr. 1988;112:948–55.
Vogtmann C, Grubbe G, Ruckhaberle KE, Bottcher H, Ockert C. Effects of early therapy with indomethacin on the manifestation of a persistent ductus arteriosus in extremely underweight premature infants. Monatsschr Kinderheilkd. 1988;136:636–9.
Bada HS, Green RS, Pourcyrous M, Leffler CW, Korones SB, Magill HL, et al. Indomethacin reduces the risks of severe intraventricular hemorrhage. J Pediatr. 1989;115:631–7.
Cassady G, Crouse DT, Kirklin JW, Strange MJ, Joiner CH, Godoy G, et al. A randomized, controlled trial of very early prophylactic ligation of the ductus arteriosus in babies who weighed 1000 g or less at birth. N Engl J Med. 1989;320:1511–6.
Krauss AN, Fatica N, Lewis BS, Cooper R, Thaler HT, Cirrincione C, et al. Pulmonary function in preterm infants following treatment with intravenous indomethacin. Am J Dis Child. 1989;143:78–81.
Lai TH, Soong WJ, Hwang B. Indomethacin for the prevention of symptomatic patent ductus arteriosus in very low birth weight infants. Zhonghua Min Guo Xiao Er Ke Yi Xue Hui Za Zhi. 1990;31:17–23.
Amato M, Huppi P, Markus D. Prevention of symptomatic patent ductus arteriosus with ethamsylate in babies treated with exogenous surfactant. J Perinatol. 1993;13:2–7.
Domanico RS, Waldman JD, Lester LA, McPhillips HA, Catrambone JE, Covert RF. Prophylactic indomethacin reduces the incidence of pulmonary hemorrhage and patent ductus arteriosus in surfactant-treated infants <1250 g. Pediatr Res. 1994;35:331A.
Ment LR, Oh W, Ehrenkranz RA, Philip AG, Vohr B, Allan W, et al. Low-dose indomethacin and prevention of intraventricular hemorrhage: a multicenter randomized trial. Pediatrics. 1994;93:543–50.
Ment LR, Oh W, Ehrenkranz RA, Phillip AG, Vohr B, Allan W, et al. Low-dose indomethacin therapy and extension of intraventricular hemorrhage: a multicenter randomized trial. J Pediatr. 1994;124:951–5.
Morales-Suarez M, Danchez-Gil T, Lemus-Varela L, Udaeta-Mora E. Estudio comparitivo de dosis baja de indometicina profilactica para hemorragia subependimaria/intraventicular en neonatos pretermino con ventilation mechanica. Bol Med Hosp Infant Mex. 1994;51:389–94.
Couser RJ, Ferrara TB, Wright GB, Cabalka AK, Schilling CG, Hoekstra RE, et al. Prophylactic indomethacin therapy in the first twenty-four hours of life for the prevention of patent ductus arteriosus in preterm infants treated prophylactically with surfactant in the delivery room. J Pediatr. 1996;128:631–7.
Yaseen H, al Umran K, Ali H, Rustum M, Darwich M, al-Faraidy A. Effects of early indomethacin administration on oxygenation and surfactant requirement in low birth weight infants. J Trop Pediatr. 1997;43:42–6.
Supapannachart S, Khowsathit P, Patchakapati B. Indomethacin prophylaxis for patent ductus arteriosus (PDA) in infants with a birth weight of less than 1250 grams. J Med Assoc Thai. 1999;82 Suppl 1:S87–92.
Dani C, Bertini G, Reali MF, Murru P, Fabris C, Vangi V, et al. Prophylaxis of patent ductus arteriosus with ibuprofen in preterm infants. Acta Paediatr. 2000;89:1369–74.
De Carolis MP, Romagnoli C, Polimeni V, Piersigilli F, Zecca E, Papacci P, et al. Prophylactic ibuprofen therapy of patent ductus arteriosus in preterm infants. Eur J Pediatr. 2000;159:364–8.
Schmidt B, Davis P, Moddemann D, Ohlsson A, Roberts RS, Saigal S, et al. Long-term effects of indomethacin prophylaxis in extremely-low-birth-weight infants. N Engl J Med. 2001;344:1966–72.
van Overmeire B, van de Broek H, van Laer P, Weyler J, Vanhaesebrouck P. Early versus late indomethacin treatment for patent ductus arteriosus in premature infants with respiratory distress syndrome. J Pediatr. 2001;138:205–11.
Gournay V, Roze JC, Kuster A, Daoud P, Cambonie G, Hascoet JM, et al. Prophylactic ibuprofen versus placebo in very premature infants: a randomised, double-blind, placebo-controlled trial. Lancet. 2004;364:1939–44.
van Overmeire B, Allegaert K, Casaer A, Debauche C, Decaluwe W, Jespers A, et al. Prophylactic ibuprofen in premature infants: a multicentre, randomised, double-blind, placebo-controlled trial. Lancet. 2004;364:1945–9.
Dani C, Bertini G, Pezzati M, Poggi C, Guerrini P, Martano C, et al. Prophylactic ibuprofen for the prevention of intraventricular hemorrhage among preterm infants: a multicenter, randomized study. Pediatrics. 2005;115:1529–35.
Sangtawesin V, Sangtawesin C, Raksasinborisut C, Sathirakul K, Kanjanapattanakul W, Khorana M, et al. Oral ibuprofen prophylaxis for symptomatic patent ductus arteriosus of prematurity. J Med Assoc Thai. 2006;89:314–21.
Sangtawesin C, Sangtawesin V, Lertsutthiwong W, Kanjanapattanakul W, Khorana M, Ayudhaya JK. Prophylaxis of symptomatic patent ductus arteriosus with oral ibuprofen in very low birth weight infants. J Med Assoc Thai. 2008;91 Suppl 3:S28–34.
Aranda JV, Clyman R, Cox B, Van Overmeire B, Wozniak P, Sosenko I, et al. A randomized, double-blind, placebo-controlled trial on intravenous ibuprofen L-lysine for the early closure of nonsymptomatic patent ductus arteriosus within 72 h of birth in extremely low-birth-weight infants. Am J Perinatol. 2009;26:235–45.
Ghanem S, Mostafa M, Shafee M. Effect of oral ibuprofen on patent ductus arteriosus in premature newborns. J Saudi Heart Assoc. 2010;22:7–12.
Kanmaz G, Erdeve O, Canpolat FE, Oguz SS, Uras N, Altug N, et al. Serum ibuprofen levels of extremely preterm infants treated prophylactically with oral ibuprofen to prevent patent ductus arteriosus. Eur J Clin Pharmacol. 2013;69:1075–81.
Kluckow M, Jeffery M, Gill A, Evans N. A randomised placebo-controlled trial of early treatment of the patent ductus arteriosus. Arch Dis Child Fetal Neonatal Ed. 2014;99:F99–104.
Harkin P, Harma A, Aikio O, Valkama M, Leskinen M, Saarela T, et al. Paracetamol accelerates closure of the ductus arteriosus after premature birth: a randomized trial. J Pediatr. 2016;177:72–7.
Kalani M, Shariat M, Khalesi N, Farahani Z, Ahmadi S. A comparison of early ibuprofen and indomethacin administration to prevent intraventricular hemorrhage among preterm infants. Acta Med Iran. 2016;54:788–92.
de Waal K, Phad N, Stubbs M, Chen Y, Kluckow M. A double blind, randomised controlled pilot trial of early pharmacological treatment versus supportive management in preterm infants with a patent ductus arteriosus (PDA). E-PAS2019:2842.345.
Hagadorn JI, Bennett MV, Brownell EA, Payton KSE, Benitz WE, Lee HC. Covariation of neonatal intensive care unit-level patent ductus arteriosus management and in-neonatal intensive care unit outcomes following preterm birth. J Pediatr. 2018;203:225–33.
Clyman RI. Patent ductus arteriosus, its treatments, and the risks of pulmonary morbidity. Semin Perinatol. 2018;42:235–42.
Liebowitz M, Katheria A, Sauberan J, Singh J, Nelson K, Hassinger DC, et al. Lack of equipoise in the PDA-TOLERATE trial: a comparison of eligible infants enrolled in the trial and those treated outside the trial. J Pediatr. 2019;213:222–6.
Author information
Authors and Affiliations
Contributions
As the corresponding author and first author, I confirm that I have full access to the data in the study and final responsibility for the decision to submit for publication. Both myself and contributing co-author, WEB MD, were involved in conceiving the work that led to the submission and played an important role in interpreting the results. I wrote the initial draft of the paper and we were both involved in revising the paper. Both approve this final revised version and we agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Corresponding author
Ethics declarations
Conflict of interest
WEB has served as a paid member of a scientific advisory panel for Abbott Laboratories, which manufactures devices for transcatheter ductal occlusion. Neither MNS nor WEB has any other potential conflicts to disclose.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Sankar, M.N., Benitz, W.E. Does crossover treatment of control subjects invalidate results of randomized trials of patent ductus arteriosus treatment?. J Perinatol 40, 1863–1870 (2020). https://doi.org/10.1038/s41372-020-00848-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41372-020-00848-z
This article is cited by
-
Evaluation of the association between patent ductus arteriosus approach and neurodevelopment in extremely preterm infants
Journal of Perinatology (2024)
-
Transcatheter closure in preterm infants with patent ductus arteriosus: feasibility, results, hemodynamic monitoring and future prospectives
Italian Journal of Pediatrics (2023)
-
The patent ductus arteriosus management debate: it’s not over yet
Journal of Perinatology (2021)