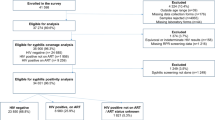
Abstract
Objectives
To examine the effect of maternal reverse-sequence (RS) syphilis screening on management of infants at risk for congenital syphilis (CS) using a standardized approach.
Study design
A retrospective study from 2011 to 2014 at an academic medical center using RS testing, involving chemiluminescent immunoassay (CIA), rapid plasma reagin (RPR), and fluorescent treponemal antibody-absorption (FTA-ABS) assays for syphilis. Clinical management and outcomes of infants born to mothers with discordant (CIA+/RPR−/FTA+) serology were compared with national or internal guidelines.
Results
Sixty-three infants were classified as discordant (n = 21), presumed false positive (CIA+/RPR−/FTA−; n = 16), or true positive (CIA+/RPR+; n = 26) based on maternal serology. Only 24% of cases in the discordant group underwent recommended full evaluation. None of the evaluated infants in the discordant group (n = 8) were diagnosed with CS.
Conclusions
Management of infants with discordant maternal RS serology remained reliant on clinical judgment. In our high-risk population, RS testing did not identify additional cases of CS.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Newman L, Kamb M, Hawkes S, Gomez G, Say L, Seuc A, et al. Global estimates of syphilis in pregnancy and associated adverse outcomes: analysis of multinational antenatal surveillance data. PLoS Med. 2013;10:e1001396.
Centers for Disease Control and Prevention. Sexually transmitted disease surveillance 2017. Atlanta; 2018.
Centers for Disease Control and Prevention. Epidemic of congenital syphilis—Baltimore, 1996–1997. MMWR Morb Mortal Wkly Rep. 1998;47:904–7.
Centers for Disease Control and Prevention. NCHHSTP AtlasPlus.Updated 2017. https://www.cdc.gov/nchhstp/atlas/index.htm. Accessed 11 Feb 2019.
Genç M, Ledger WJ. Syphilis in pregnancy. Sex Trasm Inf. 2000;76:73–79.
Park IU, Chow JM, Bolan G, Stanley M, Shieh J, Schapiro JM. Screening for syphilis with the treponemal immunoassay: analysis of discordant serology results and implications for clinical management. J Infect Dis. 2011;204:1297–304.
Mmeje O, Chow JM, Davidson L, Shieh J, Schapiro JM, Park IU. Discordant syphilis immunoassays in pregnancy: perinatal outcomes and implications for clinical management. Clin Infect Dis. 2015;61:1049–53.
Workowski KA, Bolan GA, Centers for Disease Control and Prevention. Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm Rep. 2015;64(RR-03):1–137.
Communicable Diseases and Related Conditions of Public Health Importance—Congenital Syphilis. Code of Maryland Regulations (COMAR) 10.06.01.17 D. 2017. http://Mdrules.Elaws.Us/Comar/10.06.01.17. Accessed 10 Jan 2019.
American Academy of Pediatrics. Syphilis. In: Kimberlin DW, Brady MT, Jackson MA, Long SS, editors. Red Book: 2018 Report of the Committee on Infectious Diseases. 31st edn. Illinois: American Academy of Pediatrics; 2018. p. 773–87.
Huh HJ, Chung JW, Park SY, Chae SL. Comparison of automated treponemal and nontreponemal test algorithms as first-line syphilis screening assays. Ann Lab Med. 2016;36:23–27.
Seña AC, White BL, Sparling PF. Novel treponema pallidum serologic tests: a paradigm shift in syphilis screening for the 21st century. Clin Infect Dis. 2010;51:700–8.
Centers for Disease Control and Prevention. Discordant results from reverse sequence syphilis screening—five laboratories, United States, 2006–2010. MMWR. 2011;60:133–7.
Hunter MG, Robertson PW, Post JJ. Significance of isolated reactive treponemal chemiluminescence immunoassay results. J Infect Dis. 2013;207:1416–23.
Sheffield JS, Wendel GD. Syphilis in pregnancy. Clin Obstet Gynecol. 1999;42:97–106.
Fiumara NJ, Fleming WL, Downing JG, Good FL. The incidence of prenatal syphilis at the Boston City Hospital. N Engl J Med. 1952;247:48–52.
American Academy of Pediatrics. Syphilis. In: Kimberlin DW, Brady MT, Jackson MA, Long SS, editors. Red Book: 2015 Report of the Committee on Infectious Diseases. 30th edn. Illinois: American Academy of Pediatrics; 2015. p. 755–68.
Rossi KQ, Nickel JR, Wissel ME, O’Shaughnessy RW. Passively acquired treponemal antibody from intravenous immunoglobulin therapy in a pregnant patient. Arch Pathol Lab Med. 2002;126:1237–8.
Constable SA, Parry CM, Enevoldson TP, Bradley M. Positive serological tests for syphilis and administration of intravenous immunoglobulin. Sex Transm Infect. 2007;83:57–58.
Peterman TA, Fakile YF. What is the use of rapid syphilis tests in the United States? Sex Transm Dis. 2016;43:201–3.
Acknowledgements
The authors would like to thank Pablo J. Sanchez, MD (Division of Neonatology, Ohio State University College of Medicine, Columbus, Ohio) for his advice during initial generation of the internal algorithm. They also thank Pranita D. Tamma, MD, MHS and Alice J. Hsu, Pharm. D. (Division of Pediatric Infectious Diseases, Johns Hopkins University School of Medicine) for updating our algorithm and for providing additional recommendations for evaluation and management. Finally, they thank Jeanne S. Sheffield, MD (Department of Gynecology and Obstetrics, Johns Hopkins University School of Medicine) for advice in revising this paper.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Chen, M.W., Akinboyo, I.C., Sue, P.K. et al. Evaluating congenital syphilis in a reverse sequence testing environment. J Perinatol 39, 956–963 (2019). https://doi.org/10.1038/s41372-019-0387-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41372-019-0387-9