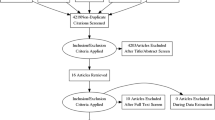
Abstract
Objective
Fetal and neonatal alloimmune thrombocytopenia (FNAIT) is associated with life-threatening bleeding. This systematic review of postnatal management of FNAIT examined transfusion of human platelet antigen (HPA) selected or unselected platelets, and/or IVIg on platelet increments, hemorrhage and mortality.
Study design
MEDLINE, EMBASE and Cochrane searches were conducted until 11 May 2018.
Result
Of 754 neonates, 382 received platelet transfusions (51%). HPA-selected platelets resulted in higher platelet increments and longer response times than HPA-unselected platelets. However, unselected platelets generally led to sufficient platelet increments to 30 × 109/L, a level above which intracranial hemorrhage or other life-threatening bleeding rarely occurred. Platelet increments were not improved with the addition of IVIg to platelet transfusion.
Conclusion
Overall, HPA-selected platelet transfusions were more effective than HPA-unselected platelets but unselected platelets were often effective enough to achieve clinical goals. Available studies do not clearly demonstrate a benefit for addition of IVIg to platelet transfusion.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Kjeldsen-Kragh J, Killie MK, Tomter G, Golebiowska E, Randen I, Hauge R, et al. A screening and intervention program aimed to reduce mortality and serious morbidity associated with severe neonatal alloimmune thrombocytopenia. Blood. 2007;110:833–9.
Turner ML, Bessos H, Fagge T, Harkness M, Rentoul F, Seymour J, et al. Prospective epidemiologic study of the outcome and cost-effectiveness of antenatal screening to detect neonatal alloimmune thrombocytopenia due to anti-HPA-1a. Transfusion. 2005;45:1945–56.
Williamson LM, Hackett G, Rennie J, Palmer CR, Maciver C, Hadfield R, et al. The natural history of fetomaternal alloimmunization to the platelet-specific antigen HPA-1a (PlA1, Zwa) as determined by antenatal screening. Blood. 1998;92:2280–7.
Arnold DM, Smith JW, Kelton JG. Diagnosis and management of neonatal alloimmune thrombocytopenia. Transfus Med Rev. 2008;22:255–67.
Delbos F, Bertrand G, Croisille L, Ansart-Pirenne H, Bierling P, Kaplan C. Fetal and neonatal alloimmune thrombocytopenia: predictive factors of intracranial hemorrhage. Transfusion. 2016;56:59–66.
Ghevaert C, Campbell K, Walton J, Smith GA, Allen D, Williamson LM, et al. Management and outcome of 200 cases of fetomaternal alloimmune thrombocytopenia. Transfusion. 2007;47:901–10.
Tiller H, Kamphuis MM, Flodmark O, Papadogiannakis N, David AL, Sainio S, et al. Fetal intracranial haemorrhages caused by fetal and neonatal alloimmune thrombocytopenia: an observational cohort study of 43 cases from an international multicentre registry. BMJ Open. 2013;3:e002490.
Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151:264–9.
Fowkes FG, Fulton PM. Critical appraisal of published research: introductory guidelines. BMJ. 1991;302:1136–40.
Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al. GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. J Clin Epidemiol. 2011;64:383–94.
Mueller-Eckhardt C, Kiefel V, Grubert A, Kroll H, Weisheit M, Schmidt S, et al. 348 cases of suspected neonatal alloimmune thrombocytopenia. Lancet. 1989;1:363–6.
Mueller-Eckhardt C, Kiefel V, Grubert A. High-dose IgG treatment for neonatal alloimmune thrombocytopenia. Blut. 1989;59:145–6.
Bussel JB, Zacharoulis S, Kramer K, McFarland JG, Pauliny J, Kaplan C. Clinical and diagnostic comparison of neonatal alloimmune thrombocytopenia to non-immune cases of thrombocytopenia. Pediatr Blood Cancer. 2005;45:176–83.
Allen D, Verjee S, Rees S, Murphy MF, Roberts DJ. Platelet transfusion in neonatal alloimmune thrombocytopenia. Blood. 2007;109:388–9.
Allen DL, Samol J, Benjamin S, Verjee S, Tusold A, Murphy MF. Survey of the use and clinical effectiveness of HPA-1a/5b-negative platelet concentrates in proven or suspected platelet alloimmunization. Transfus Med. 2004;14:409–17.
Bakchoul T, Bassler D, Heckmann M, Thiele T, Kiefel V, Gross I, et al. Management of infants born with severe neonatal alloimmune thrombocytopenia: the role of platelet transfusions and intravenous immunoglobulin. Transfusion. 2014;54:640–5.
Fratellanza G, Fratellanza A, Paesano L, Scarcella A, Safoian A, Misso S, et al. Fetoneonatal alloimmune thrombocytopenia (FNAIT): our experience. Transfus Apher Sci. 2006;35:111–7.
Galea P, Patrick MJ, Goel KM. Isoimmune neonatal thrombocytopenic purpura. Arch Dis Child. 1981;56:112–5.
Glade-Bender J, McFarland JG, Kaplan C, Porcelijn L, Bussel JB. Anti-HPA-3A induces severe neonatal alloimmune thrombocytopenia. J Pediatr. 2001;138:862–7.
Kiefel V, Bassler D, Kroll H, Paes B, Giers G, Ditomasso J, et al. Antigen-positive platelet transfusion in neonatal alloimmune thrombocytopenia (NAIT). Blood. 2006;107:3761–3.
Lee K, Beaujean F, Bierling P. Treatment of severe fetomaternal alloimmune thrombocytopenia with compatible frozen-thawed platelet concentrates. Br J Haematol. 2002;117:482–3.
te Pas AB, Lopriore E, van den Akker ES, Oepkes D, Kanhai HH, Brand A, et al. Postnatal management of fetal and neonatal alloimmune thrombocytopenia: the role of matched platelet transfusion and IVIG. Eur J Pediatr. 2007;166:1057–63.
Crighton GL, Scarborough R, McQuilten ZK, Phillips LE, Savoia HF, Williams B, et al. Contemporary management of neonatal alloimmune thrombocytopenia: good outcomes in the intravenous immunoglobulin era: results from the Australian neonatal alloimmune thrombocytopenia registry. J Matern Fetal Neonatal Med. 2017;30:2488–94.
Singh-Grewal D, Kemp A, Wong M. A prospective study of the immediate and delayed adverse events following intravenous immunoglobulin infusions. Arch Dis Child. 2006;91:651–4.
Krishnan L, Pathare A. Necrotizing enterocolitis in a term neonate following intravenous immunoglobulin therapy. Indian J Pediatr. 2011;78:743–4.
Luban NL, Wong EC, Henrich Lobo R, Pary P, Duke S. Intravenous immunoglobulin-related hemolysis in patients treated for Kawasaki disease. Transfusion. 2015;55(Suppl 2):S90–4.
Akman AO, Kara FK, Koksal T, Cakir BC, Karagol C, Sayli T. Association of hemolysis with high dose intravenous immunoglobulin therapy in pediatric patients: an open-label prospective trial. Transfus Apher Sci. 2017;56:531–4.
Khan S, Dore PC, Sewell WA. Intravenous immunoglobulin-induced neutropenia. Pediatr Allergy Immunol. 2010;21:892–3.
Majer RV, Green PJ. Neutropenia caused by intravenous immunoglobulin. Br Med J. 1988;296:1262.
Veys PA, Macey MG, Owens CM, Newland AC. Neutropenia following intravenous immunoglobulin. Br Med J. 1988;296:1800.
Acknowledgements
We thank Elizabeth Uleryk for the search strategy; and Sylvia Torrance and Kimberly Figures for administrative assistance.
International Collaboration for Transfusion Medicine Guidelines (ICTMG)
Shubha Allard, MD, FRCP, FRCP(Path), Barts Health NHS Trust and NHS Blood & Transplant, UK; Celso Bianco, MD, formerly with America’s Blood Centres, USA; Jeannie Callum, BA, MD, FRCPC, CTBS, University of Toronto, Canada; Veerle Compernolle, MD, PhD, Belgian Red Cross, Flanders, Belgium; Dean Fergusson, MHA, PhD, University of Ottawa, Canada; Mark Fung, MD, PhD, University of Vermont Medical Center, USA; Susan Nahirniak, MD, FRCPC, University of Alberta, Canada; Katerina Pavenski, MD, FRCPC, University of Toronto, Canada; Joanne Pink, MBBS, FRACP, FRCPA, Australian Red Cross Blood Service, Australia; Arjuna Ponnampalam, MD, FRCPC, University of Manitoba, Canada; Paolo Rebulla, Ospedale Maggiore Policlinico, Italy; Cynthia So-Osman, Sanquin, Netherlands; Simon J. Stanworth, MA, MRCP, DPhil, FRCPath, University of Oxford, UK; Zbigniew M. Szczepiorkowski, MD, PhD, Dartmouth-Hitchcock Medical Centre, USA; Alan T. Tinmouth, MD, FRCPC, MSc, University of Ottawa, Canada; Erica Wood, MBBS, FRACP, FRCPA, Monash University, Australia.
Author contributions
JMB reviewed the citations, analyzed data, drafted the initial manuscript, and participated in critical revisions of the article. HH reviewed the citations, analyzed data, drafted the initial manuscript, and participated in critical revisions of the article. NS reviewed the citations, extracted data and supported all drafts of the manuscript. ST extracted data and supported all drafts of the manuscript. DL finalized the tables and figures, participated in the interpretation of data, and critical revisions of the article. TB, LL, and EM reviewed citations, participated in the interpretation of data, and critical revisions of the article. JB, MFM, AG, DMA, SB, GB, MK, CK, JK-K, DO, HS and GR participated in the interpretation of data and critical revisions of the article. All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
Funding
Canadian Blood Services partially funded this study. Canadian Blood Services did not have any role in the design, analysis, and interpretation of the data or preparation, review, and approval of the manuscript.
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Conflict of interest
MK and JK-K are two of the founders and owners of Prophylix Pharma AS, a Norwegian biotech company coordinating the EU-funded PROFNAIT Consortium which is developing a prophylaxis against fetal and neonatal alloimmune thrombocytopenia (FNAIT). DO has received research funding to a project “Towards routine HPA-screening in Pregnancy”. NS and DMA are consultants for Canadian Blood Services. J Bussel is a consultant of Baxalta, Superior Biologics and Prophylix Pharma. The remaining authors report no conflict of interest.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Members of the International Collaboration for Transfusion Medicine Guidelines (ICTMG) are listed below the Acknowledgements.
Supplementary information
Rights and permissions
About this article
Cite this article
Baker, J.M., Shehata, N., Bussel, J. et al. Postnatal intervention for the treatment of FNAIT: a systematic review. J Perinatol 39, 1329–1339 (2019). https://doi.org/10.1038/s41372-019-0360-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41372-019-0360-7