Abstract
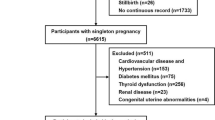
The objectives of this study were 1—to evaluate the prevalence of masked chronic hypertension in pregnant women classified as gestational hypertension 2—to compare the risks of developing preeclampsia in true gestational hypertension vs those women classified as having gestational hypertension but who had had masked hypertension in the first half of pregnancy. We conducted a cohort study in consecutive high-risk pregnancies who were evaluated before 20 weeks of gestation. Women who developed gestational hypertension (normotension in the office before 20 weeks of gestation and office BP ≥ 140/90 mmHg and/or antihypertensive treatment in the second half of gestation) were divided, according to an ABPM performed before 20 weeks of pregnancy, in two subgroups: subgroup 1—if their ABPM was normal, and subgroup 2—if they had masked chronic hypertension. Risks for preeclampsia (PE) were estimated and compared with normotensive women. Before 20 weeks of gestation, 227 women were evaluated (age 32 ± 6 years, median gestation age 15 weeks); 67 had chronic hypertension (29.5%). Of the remaining 160, 39 developed gestational hypertension (16 in subgroup 1 and 23 insubgroup 2. Compared with normotensive pregnant women, subgroup 1 of women with gestational hypertension did not increase the risk of developing PE (OR = 0.76, 95% CI = 0.16–6.65). Conversely, subgroup 2 of gestational hypertension increased the risk of PE more than 4 times (0R = 4.47 CI = 1.16–12.63). Risk estimation did not change substantially after the adjustment for multiple possible confounders. In conclusion, the59% of women initially diagnosed as gestational hypertensive according to current recommendations had masked chronic hypertension and a very high risk of developing PE.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
Data availability
The authors have available the databases and results of the studies that are kept in the Cardiometabolic Diseases Unit of the Hospital San Martin de La Plata.
References
Gillon TE, Pels A, von Dadelszen P, MacDonell K, Magee LA. Hypertensive disorders of pregnancy: a systematic review of international clinical practice guidelines. PLoS ONE. 2014;9:e113715.
Garovic VD, Dechend R, Easterling T, Karumanchi SA, McMurtry Baird S, Magee LA. et al. American Heart Association Council on Hypertension; Council on the Kidney in Cardiovascular Disease, Kidney in Heart Disease Science Committee; Council on Arteriosclerosis, Thrombosis and Vascular Biology; Council on Lifestyle and Cardiometabolic Health; Council on Peripheral Vascular Disease; and Stroke Council. Hypertension in pregnancy: diagnosis, blood pressure goals, and pharmacotherapy: a scientific statement From the American Heart Association. Hypertension. 2022;79:e21–e41.
Brown MA, Magee LA, Kenny LC, Karumanchi SA, McCarthy FP, Saito S, et al. International Society for the Study of Hypertension in Pregnancy (ISSHP). The hypertensive disorders of pregnancy: ISSHP classification, diagnosis & management recommendations for international practice. Hypertension. 2018;13:291–310.
Salazar MR, Espeche WG, Balbín E, Leiva Sisnieguez CE, Leiva Sisnieguez BC, Stavile RN, et al. Office blood pressure values and the necessity of out-of-office measurements in high-risk pregnancies. J Hypertens. 2019;37:1838–44.
Bakker R, Steegers EA, Hofman A, Jaddoe VW. Blood pressure in different gestational trimesters, fetal growth, and the risk of adverse birth outcomes: the generation R study. Am J Epidemiol. 2011;174:797–806.
Wu P, Chew-Graham CA, Maas AH, Chappell LC, Potts JE, Gulati M, et al. Temporal changes in hypertensive disorders of pregnancy and impact on cardiovascular and obstetric outcomes. Am J Cardiol. 2020;125:1508–16.
Saudan P, Brown MA, Buddle ML, Jones M. Does gestational hypertension become pre-eclampsia? Br J Obstet Gynaecol. 1998;105:1177–84.
Davis GK, Mackenzie C, Brown MA, Homer CS, Holt J, McHugh L, et al. Predicting transformation from gestational hypertension to preeclampsia in clinical practice: a possible role for 24 h ambulatory blood pressure monitoring. Hypertens Pregnancy. 2007;26:77–87.
Salazar MR, Espeche WG, Leiva Sisnieguez BC, Balbín E, Leiva Sisnieguez CE, Stavile RN, et al. Significance of masked and nocturnal hypertension in normotensive women coursing a high-risk pregnancy. J Hypertens. 2016;34:2248–52.
Gupta M, Shennan AH, Halligan A, Taylor DJ, de Swiet M. Accuracy of oscillometric blood pressure monitoring in pregnancy and pre-eclampsia. Br J Obstet Gynaecol. 1997;104:350–5. https://doi.org/10.1111/j.1471-0528.1997.tb11467.x.
Brown MA, Buddle ML, Bennett M, Smith B, Morris R, Whitworth JA. Ambulatory blood pressure in pregnancy: comparison of the Spacelabs 90207 and Accutracker II monitors with intraarterial recordings. Am J Obstet Gynecol. 1995;173:218–23.
Bello NA, Woolley JJ, Cleary KL, Falzon L, Alpert BS, Oparil S, et al. Accuracy of blood pressure measurement devices in pregnancy: a systematic review of validation studies. Hypertension. 2018;71:326–35.
Tita AT, Szychowski JM, Boggess K, Dugoff L, Sibai B, Lawrence K, et al. Chronic Hypertension and Pregnancy (CHAP) Trial Consortium. Treatment for mild chronic hypertension during pregnancy. N Engl J Med. 2022;386:1781–92.
Wu DD, Gao L, Huang O, Ullah K, Guo MX, Liu Y, et al. Increased adverse pregnancy outcomes associated with stage 1 hypertension in a low-risk cohort: Evidence from 47 874 cases. Hypertension. 2020;75:772–80.
Wikström AK, Haglund B, Olovsson M, Lindeberg SN. The risk of maternal ischaemic heart disease after gestational hypertensive disease. BJOG. 2005;112:1486–91.
Theilen LH, Fraser A, Hollingshaus MS, Schliep KC, Varner MW, Smith KR, et al. All-cause and cause-specific mortality after hypertensive disease of pregnancy. Obstet Gynecol. 2016;128:238–44.
Banegas JR, Ruilope LM, de la Sierra A, Vinyoles E, Gorostidi M, de la Cruz JJ. et al. Relationship between clinic and ambulatory blood-pressure measurements and mortality. N Engl J Med. 2018;378:1509–20.
Lv LJ, Ji WJ, Wu LL, Miao J, Wen JY, Lei Q, et al. Thresholds for ambulatory blood pressure monitoring based on maternal and neonatal outcomes in late pregnancy in a southern Chinese population. J Am Heart Assoc. 2019;8:e012027.
Acknowledgements
We acknowledge Luz Salazar Landea and María Carolina Ferrari for the final English corrections.
Author information
Authors and Affiliations
Contributions
Participation in patient care: AS, FG, CS, ST, and JM. Data upload and track records: GC, PCR, and EB. Statistical analysis: RS, CLS. Revision of the writing: HC. Original idea, coordination and elaboration of the writing: MS and WE.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
The study protocol was approved by the medical bioethics committee of the Faculty of Medical Sciences, National University of La Plata (UNLP), Buenos Aires, Argentina (COBIMED 0/27).
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Espeche, W.G., Salazar, M.R., Minetto, J. et al. Hypertension arising after 20 weeks of gestation: gestational hypertension or masked chronic hypertension?. J Hum Hypertens 37, 813–817 (2023). https://doi.org/10.1038/s41371-022-00767-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41371-022-00767-w
This article is cited by
-
Mitigating preeclampsia risk through effective uncontrolled blood pressure management
Hypertension Research (2024)
-
Uncontrolled and masked uncontrolled blood pressure in treated pregnant women with chronic hypertension and risk for preeclampsia/eclampsia
Hypertension Research (2023)