Abstract
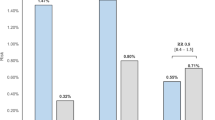
A model-based meta-analysis quantified comparative dyskalemia risk (hyper- or hypo-kalemia) in hypertensive patients treated with angiotensin receptor blockers (ARBs), a calcium channel blocker (CCB) and/or a thiazide diuretic (hydrochlorothiazide; HCTZ) as monotherapy or as fixed-dose combinations. Among 15 randomized controlled trials in a US Food and Drug Administration regulatory review database, dyskalemia events were reported by five trials (24 treatment arms, 11,030 subjects, 8-week median follow up time). The five trials evaluated monotherapy (ARB or HCTZ) alongside dual (ARB + HCTZ, ARB + CCB, or HCTZ + CCB) or triple fixed-dose combinations (ARB + CCB + HCTZ). Hypo- and hyper-kalemia rates were analyzed jointly to account for correlation. Significant drug class, drug, or dose effects were included in the final model. Effect on various drug- and dose combinations on dyskalemia risk were simulated and compared with model-estimated placebo arm dyskalemia risk. After a typical follow-up of 8 weeks, fixed-dose combinations of ARB with a high dose (25 mg) of HCTZ were associated with a higher hypokalemia risk difference (RD) from placebo (e.g.,Valsartan + HCTZ: 2.52%[95%CIs:1.17, 4.38%]). However, when ARB was combined with a lower, 12.5 mg dose of HCTZ, hypokalemia RD from placebo was not significant (Valsartan + HCTZ: −0.03%[−0.80, 0.71%]). ARB monotherapy raised hyperkalemia RD from placebo (1.3%[0.3, 3.6%]). Hyperkalemia risk was not appreciably higher than placebo for any FDC that combined ARB with HCTZ (Valsartan + HCTZ: 0.06%[−1.48, 1.64%]). In uncomplicated hypertensive patients, ARB + 12.5 mg HCTZ fixed-dose combinations are safer with respect to dyskalemia than either ARB or HCTZ monotherapy for initial antihypertensive treatment.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Unger T, Borghi C, Charchar F, Khan NA, Poulter NR, Prabhakaran D, et al. 2020 International Society of Hypertension Global Hypertension Practice Guidelines. Hypertension. 2020;75:1334–57.
Wald DS, Law M, Morris JK, Bestwick JP, Wald NJ. Combination therapy versus monotherapy in reducing blood pressure: meta-analysis on 11,000 participants from 42 trials. Am J Med. 2009;122:290–300.
Salam A, Huffman MD, Kanukula R, Hari Prasad E, Sharma A, Heller DJ, et al. Two-drug fixed-dose combinations of blood pressure-lowering drugs as WHO essential medicines: an overview of efficacy, safety, and cost. J Clin Hypertens. 2020;22:1769–79.
Papademetriou V. Diuretics, hypokalemia, and cardiac arrhythmia: a 20-year controversy. J Clin Hypertens. 2006;8:86–92.
Ellison DH, Loffing J. Thiazide effects and adverse effects: insights from molecular genetics. Hypertension. 2009;54:196–202.
Alderman MH, Piller LB, Ford CE, Probstfield JL, Oparil S, Cushman WC, et al. Clinical significance of incident hypokalemia and hyperkalemia in treated hypertensive patients in the antihypertensive and lipid-lowering treatment to prevent heart attack trial. Hypertension. 2012;59:926–33.
Raebel MA. Hyperkalemia associated with use of angiotensin-converting enzyme inhibitors and angiotensin receptor blockers. Cardiovasc Ther. 2012;30:e156–e166.
Maringwa J, Sardu ML, Hang Y, Czerniak R, Vishnubhotla M, Vakilynejad M, et al. Characterizing effects of antidiabetic drugs on heart rate, systolic and diastolic blood pressure. Clin Pharmacol Ther. 2021;109:1583–92.
Plock N, Bax L, Lee D, DeManno D, Lahu G, Pfister M. Exploratory literature meta-analysis to characterize the relationship between early and longer term body weight loss for antiobesity compounds. J Clin Pharm. 2017;57:52–63.
Naik H, Lu J, Cao C, Pfister M, Vakilynejad M, Leifke E. Pharmacometric approaches to guide dose selection of the novel GPR40 agonist TAK-875 in subjects with type 2 diabetes mellitus. CPT Pharmacomet Syst Pharm. 2013;2:e22.
Mandema JW, Gibbs M, Boyd RA, Wada DR, Pfister M. Model-based meta-analysis for comparative efficacy and safety: application in drug development and beyond. Clin Pharm Ther. 2011;90:766–9.
Bandak G, Sang Y, Gasparini A, Chang AR, Ballew SH, Evans M, et al. Hyperkalemia after initiating renin-angiotensin system blockade: the Stockholm Creatinine Measurements (SCREAM) Project. J Am Heart Assoc. 2017;6.
Oparil S, Melino M, Lee J, Fernandez V, Heyrman R. Triple therapy with olmesartan medoxomil, amlodipine besylate, and hydrochlorothiazide in adult patients with hypertension: the TRINITY multicenter, randomized, double-blind, 12-week, parallel-group study. Clin Ther. 2010;32:1252–69.
Daiichi Sankyo Inc. Study of co-administration of olmesartan medoxomil plus amlodipine in patients with mild to severe hypertension. 2005. [Accessed 2020; ClinicalTrials.gov identifier: NCT00185133].
Daiichi Sankyo Inc. Olmesartan as an add-on to amlodipine in hypertension. 2005. [Accessed 2020; ClinicalTrials.gov identifier: NCT00220233].
Daiichi Sankyo Inc. Azor (amlodipine and olmesartan medoxomil) 5/20, 5/40, 10/20, and 10/40 mg tablets, medical reviews NDA22-100. In: FDA, 2007. [Accessed 2020; https://www.accessdata.fda.gov/drugsatfda_docs/nda/2007/022100_azor_toc.cfm
Novartis. Clinical & Statistical Review NDA 22-314. In: FDA, 2009. Accessed 2020; https://www.accessdata.fda.gov/drugsatfda_docs/nda/2009/022314s000_MedR.pdf.
Benz JR, Black HR, Graff A, Reed A, Fitzsimmons S, Shi Y. Valsartan and hydrochlorothiazide in patients with essential hypertension. A multiple dose, double-blind, placebo controlled trial comparing combination therapy with monotherapy. J Hum Hypertens. 1998;12:861–6.
Daiichi Sankyo Inc. Safety and efficacy study of a triple combination therapy in subjects with hypertension. 2010. [Accessed 2020; ClinicalTrials.gov identifier: NCT00649389].
Franse LV, Pahor M, Di Bari M, Somes GW, Cushman WC, Applegate WB. Hypokalemia associated with diuretic use and cardiovascular events in the Systolic Hypertension Elder Program. Hypertension. 2000;35:1025–30.
Psaty BM, Smith NL, Siscovick DS, Koepsell TD, Weiss NS, Heckbert SR, et al. Health outcomes associated with antihypertensive therapies used as first-line agents. A systematic review and meta-analysis. JAMA. 1997;277:739–45.
Peterzan MA, Hardy R, Chaturvedi N, Hughes AD. Meta-analysis of dose-response relationships for hydrochlorothiazide, chlorthalidone, and bendroflumethiazide on blood pressure, serum potassium, and urate. Hypertension. 2012;59:1104–9.
Parikh RV, Nash DM, Brimble KS, Markle-Reid M, Tan TC, McArthur E, et al. Kidney function and potassium monitoring after initiation of renin-angiotensin-aldosterone system blockade therapy and outcomes in 2 north American populations. Circ Cardiovasc Qual Outcomes. 2020;13:e006415.
Kovesdy CP, Matsushita K, Sang Y, Brunskill NJ, Carrero JJ, Chodick G, et al. Serum potassium and adverse outcomes across the range of kidney function: a CKD Prognosis Consortium meta-analysis. Eur Heart J. 2018;39:1535–42.
Funding
This study was supported by Resolve to Save Lives, which is funded by Bloomberg Philanthropies, the Bill & Melinda Gates Foundation, and Gates Philanthropy Partners, which is funded with support from the Chan Zuckerberg Foundation.
Author information
Authors and Affiliations
Contributions
L.Q., N.Z., J.I., E.R.M., M.P., A.E.M., and E.C. wrote the manuscript; L.Q., N.Z., A.E.M., and E.C. designed the research; L.Q., N.Z., and E.C. performed the research; L.Q., and E.C. analyzed the data.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Qin, L., Zhang, N., Ishigami, J. et al. Dyskalemia risk associated with fixed-dose anti-hypertensive medication combinations. J Hum Hypertens 36, 989–995 (2022). https://doi.org/10.1038/s41371-021-00600-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41371-021-00600-w
This article is cited by
-
Model-Based Meta-Analysis Supporting the Combination of Acetaminophen and Topical Diclofenac in Acute Pain: A Therapy for Mild-to-Moderate Osteoarthritis Pain?
Pain and Therapy (2024)
-
Model-Based Assessment of the Liver Safety Profile of Acetaminophen to Support its Combination Use with Topical Diclofenac in Mild-to-Moderate Osteoarthritis Pain
Pain and Therapy (2024)
-
Use of Perindopril Arginine/Indapamide/Amlodipine in the Management of Hypertension in Two Sub-Saharan African Island Countries of Madagascar and Mauritius
Advances in Therapy (2022)