Abstract
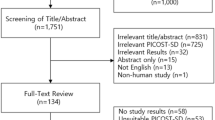
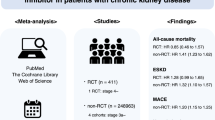
Angiotensin-converting enzyme inhibitors and angiotensin receptor blockers are drugs commonly used for the treatment of hypertension. However, studies on their comparative efficacy have not been extensively investigated. The current systematic review and network meta-analysis studied the comparative efficacy of the two antihypertensive treatment categories in reducing blood pressure, mortality, and morbidity in essential hypertension patients. A literature search was carried out in Medline and Cochrane Central Register of Controlled Trials for placebo- and active-controlled, double-blind randomized clinical trials, which had reported blood pressure effects, mortality, and/or morbidity. Blood pressure results were found in 30 studies with 7370 participants and 8 studies with 25,158 participants with mortality/morbidity results included in the analysis. The two drug classes had similar effectiveness in lowering systolic (weighted mean difference (WMD): 0.59, 95% CI: −0.21 to 1.38) and diastolic blood pressure (WMD: 0.62, 95% CI: −0.06 to 1.30), all-cause mortality (risk ratio (RR)): 0.96, 95% CI 0.80 to 1.14), cardiovascular mortality (RR: 0.87, 95% CI 0.67 to 1.14), fatal and non-fatal myocardial infarction (RR: 1.02, 95% CI 0.75 to 1.37) and stroke (RR: 1.13, 95% CI 0.87 to 1.46). Angiotensin-converting enzyme inhibitors were more helpful in the prevention and/or the hospitalization for heart failure than angiotensin receptor blockers (RR: 0.71, 95% CI 0.54 to 0.93). Angiotensin-converting enzyme inhibitors and angiotensin receptor blockers were similarly effective in decreasing blood pressure, mortality, and morbidity in essential hypertension. Angiotensin-converting enzyme inhibitors were more protective in the advancement and/or hospitalization of the hypertensive patient for heart failure than angiotensin receptor blockers.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
World Health Organization, Regional Office for Europe. High blood pressure - country experiences and effective interventions utilized across the European Region. Copenhagen: WHO Regional Office for Europe; 2013.
Bommer WJ. Use of angiotensin-converting enzyme inhibitor/angiotensin receptor blocker therapy to reduce cardiovascular events in high-risk patients: part 2. Prev Cardiol. 2008;11:215–22.
Heran BS, Wong MM, Heran IK, Wright JM. Blood pressure lowering efficacy of angiotensin converting enzyme (ACE) inhibitors for primary hypertension. Cochrane Database Syst Rev. 2008:CD003823.
Heran BS, Wong MM, Heran IK, Wright JM. Blood pressure lowering efficacy of angiotensin receptor blockers for primary hypertension. Cochrane Database Syst Rev. 2008:CD003822.
Li EC, Heran BS, Wright JM. Angiotensin converting enzyme (ACE) inhibitors versus angiotensin receptor blockers for primary hypertension. Cochrane Database Syst Rev. 2014:CD009096.
Matchar DB, McCrory DC, Orlando LA, Patel MR, Patel UD, Patwardhan MB, et al. Systematic review: comparative effectiveness of angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers for treating essential hypertension. Ann Intern Med. 2008;148:16–29.
Powers BJ, Coeytaux RR, Dolor RJ, Hasselblad V, Patel UD, Yancy WSJ, et al. Updated report on comparative effectiveness of ACE inhibitors, ARBs, and direct renin inhibitors for patients with essential hypertension: much more data, little new information. J Gen Intern Med. 2012;27:716–29.
Zou Ζ, Xi GL, Yuan HB, Zhu QF, Shi XY. Telmisartan versus angiotension-converting enzyme inhibitors in the treatment of hypertension: a meta-analysis of randomized controlled trials. J Hum Hypertens. 2009;23:339–49.
Tsoi B, Akioyamen LE, Bonner A, Frankfurter C, Levine M, Pullenayegum E, et al. Comparative efficacy of angiotensin II antagonists in essential hypertension: systematic review and network meta-analysis of randomised controlled trials. Heart Lung Circ. 2017. https://doi.org/10.1016/j.hlc.2017.06.721.
Ricci F, Di Castelnuovo A, Savarese G, Perrone Filardi P, De Caterina R. ACE-inhibitors versus angiotensin receptor blockers for prevention of events in cardiovascular patients without heart failure - a network meta-analysis. Int J Cardiol. 2016;15:128–34.
Hutton B, Salanti G, Caldwell DM, Chaimani A, Schmid CH, Cameron C, et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Intern Med. 2015;162:777–84.
Higgins JPT, Green S. Chapter 9: Analysing data and undertaking meta-analyses. In: Higgins JPT, Green S, editors. Cochrane handbook for systematic reviews of interventions. Version 5.1.0 (updated 2011). (The Cochrane Collaboration, Great Britain, 2008).
Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928.
Higgins JPT Green S. Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 [updated March 2011]. http://handbook.cochrane.org. (The Cochrane Collaboration, Great Britain, 2011).
Rücker G, Schwarzer G. Ranking treatments in frequentist network meta-analysis works without resampling methods. BMC Med Res Methodol. 2015;15:58.
Dias S, Welton NJ, Caldwell DM, Ades AE. Checking consistency in mixed treatment comparison meta-analysis. Stat Med. 2010;29:932–44.
Schünemann H, Brożek J, Guyatt G, Oxman A, editors. GRADE Handbook. Introduction to GRADE Handbook. Handbook for grading the quality of evidence and the strength of recommendations using the GRADE approach Updated October 2013. Available from http://gdt.guidelinedevelopment.org.
Puhan MA, Schünemann HJ, Murad MH, Li T, Brignardello-Petersen R, Singh JA, et al. A GRADE Working Group approach for rating the quality of treatment effect estimates from network meta-analysis. BMJ. 2014;349:g5630. https://doi.org/10.1136/bmj.g5630
Agabiti-Rosei E, Manolis A, Zava D, Omboni S. Zofenopril plus hydrochlorothiazide and irbesartan plus hydrochlorothiazide in previously treated and uncontrolled diabetic and non-diabetic essential hypertensive patients. Adv Ther. 2014;31:217–33.
Azizi M, Linhart A, Alexander J, Goldberg A, Menten J, Sweet C, et al. Pilot study of combined blockade of the renin-angiotensin system in essential hypertensive patients. J Hypertens. 2000;18:1139–47.
Brown NJ, Kumar S, Painter CA, Vaughan DE. ACE inhibition versus angiotensin type 1 receptor antagonism: differential effects on PAI-1 over time. Hypertension. 2002;40:859–65.
Chen JH, Cheng JJ, Chen CY, Chiou HC, Huang TY, Tsai CD, et al. Comparison of the efficacy and tolerability of telmisartan 40 mg vs. enalapril 10 mg in the treatment of mild-to-moderate hypertension: a multicentre, double-blind study in Taiwanese patients. Int J Clin Pract Suppl. 2004;58:29–34.
Coca A, Calvo C, Garcia-Puig J, Gil-Extremera B, Aguilera MT, de la Sierra A, et al. A multicenter, randomized, double-blind comparison of the efficacy and safety of irbesartan and enalapril in adults with mild to moderate essential hypertension, as assessed by ambulatory blood pressure monitoring: the MAPAVEL Study (Monitorizacion Ambulatoria Presion Arterial APROVEL). Clin Ther. 2002;24:126–38.
Conlin PR, Moore TJ, Swartz SL, Barr E, Gazdick L, Fletcher C, et al. Effect of indomethacin on blood pressure lowering by captopril and losartan in hypertensive patients. Hypertension. 2000;36:461–5.
De Rosa ML, Cardace P, Rossi M, Baiano A, de Cristofaro A, Rosa ML, et al. Comparative effects of chronic ACE inhibition and AT1 receptor blocked losartan on cardiac hypertrophy and renal function in hypertensive patients. J Hum Hypertens. 2002;16:133–40.
Fogari R, Mugellini A, Zoppi A, Corradi L, Preti P, Lazzari P, et al. Losartan and perindopril effects on plasma plasminogen activator inhibitor-1 and fibrinogen in hypertensive type 2 diabetic patients. Am J Hypertens. 2002;15:316–20.
Fogari R, Zoppi A, Preti P, Fogari E, Malamani G, Mugellini A. Differential effects of ACE-inhibition and angiotensin II antagonism on fibrinolysis and insulin sensitivity in hypertensive postmenopausal women. Am J Hypertens. 2001;14:921–6.
Himmelmann A, Keinänen-Kiukaanniemi S, Wester A, Redón J, Asmar R, Hedner T, et al. The effect duration of candesartan cilexetil once daily, in comparison with enalapril once daily, in patients with mild to moderate hypertension. Blood Press. 2001;10:43–51.
Holwerda NJ, Fogari R, Angeli P, Porcellati C, Hereng C, Oddou-Stock P, et al. Valsartan, a new angiotensin II antagonist for the treatment of essential hypertension: efficacy and safety compared with placebo and enalapril. J Hypertens. 1996;14:1147–51.
Leonetti G, Rappelli A, Omboni S, on behalf of the Study Group. A similar 24-h blood pressure control is obtained by zofenopril and candesartan in primary hypertensive patients. Blood Press. 2006;15:18–26.
Leu HB, Charng MJ, Ding PY. A double blind randomized trial to compare the effects of eprosartan and enalapril on blood pressure, platelets, and endothelium function in patients with essential hypertension. Jpn Heart J. 2004;45:623–35.
Malacco E, Omboni S, Parati G. Blood pressure response to zofenopril or irbesartan each combined with hydrochlorothiazide in high-risk hypertensives uncontrolled by monotherapy: a randomized, double-blind, controlled, parallel group, noninferiority trial. Int J Hypertens. 2015;2015:139465.
Malacco E, Omboni S, Volpe M, Auteri A, Zanchetti A, Grp ES. Antihypertensive efficacy and safety of olmesartan medoxomil and ramipril in elderly patients with mild to moderate essential hypertension: the ESPORT study. J Hypertens. 2010;28:2342–50.
Malacco E, Santonastaso M, Varì NA, Gargiulo A, Spagnuolo V, Bertocchi F, et al. Comparison of valsartan 160 mg with lisinopril 20 mg, given as monotherapy or in combination with a diuretic, for the treatment of hypertension: the Blood Pressure Reduction and Tolerability of Valsartan in Comparison with Lisinopril (PREVAIL) study. Clin Ther. 2004;26:855–65.
Mallion JM, Bradstreet DC, Makris L, Goldberg AI, Halasz S, Sweet CS, et al. Antihypertensive efficacy and tolerability of once daily losartan potassium compared with captopril in patients with mild to moderate essential hypertension. J Hypertens Suppl. 1995;13:S35–41.
Mallion JM, Omboni S, Barton J, Van Mieghem W, Narkiewicz K, Panzer PK, et al. Antihypertensive efficacy and safety of olmesartan and ramipril in elderly patients with mild to moderate systolic and diastolic essential hypertension. Blood Press Suppl. 2011;1:3–11.
McInnes GT, O’Kane KP, Istad H, Keinanen-Kiukaanniemi S, Van Mierlo HF, Keinänen-Kiukaanniemi S, et al. Comparison of the AT1-receptor blocker, candesartan cilexetil, and the ACE inhibitor, lisinopril, in fixed combination with low dose hydrochlorothiazide in hypertensive patients. J Hum Hypertens. 2000;14:263–9.
Modesti PA, Omboni S, Taddei S, Ghione S, Portaluppi F, Pozzilli P, et al. Zofenopril or irbesartan plus hydrochlorothiazide in elderly patients with isolated systolic hypertension untreated or uncontrolled by previous treatment: a double-blind, randomized study. J Hypertens. 2016;34:576–87.
Nalbantgil I, Nalbantgil S, Ozerkan F, Yilmaz H, Gurgun C, Zoghi M, et al. The efficacy of telmisartan compared with perindopril in patients with mild-to-moderate hypertension. Int J Clin Pract Suppl. 2004;58:50–4.
Narkiewicz K. Comparison of home and office blood pressure in hypertensive patients treated with zofenopril or losartan. Blood Press Suppl. 2007;2:7–12.
Palma Gámiz JL, Pêgo M, Contreras EM, Anglada MP, Martínez JO, Esquerra EA, et al. A twelve-week, multicenter, randomized, double-blind, parallel-group, noninferiority trial of the antihypertensive efficacy and tolerability of imidapril and candesartan in adult patients with mild to moderate essential hypertension: the Iberian Multicenter Imidapril Study on Hypertension (IMISH). Clin Ther. 2006;28:2040–51.
Rosei EA, Rizzoni D, Muiesan ML, Sleiman I, Salvetti M, Monteduro C, et al. Effects of candesartan cilexetil and enalapril on inflammatory markers of atherosclerosis in hypertensive patients with non-insulin-dependent diabetes mellitus. J Hypertens. 2005;23:435–44.
Scaglione R, Argano C, Chiara T, Parrinello G, Colomba D, Avellone G, et al. Effect of dual blockade of renin-angiotensin system on TGFbeta1 and left ventricular structure and function in hypertensive patients. J Hum Hypertens. 2007;21:307–15.
Scaglione R, Argano C, Corrao S, Di Chiara T, Licata A, Licata G, et al. Transforming growth factor beta1 and additional renoprotective effect of combination ACE inhibitor and angiotensin II receptor blocker in hypertensive subjects with minor renal abnormalities: a 24-week randomized controlled trial. J Hypertens. 2005;23:657–64.
Sega R. Efficacy and safety of eprosartan in severe hypertension. Eprosartan Multinational Study Group. Blood Press. 1999;8:114–21.
Tikkanen I, Omvik P, Jensen HA. Comparison of the angiotensin II antagonist losartan with the angiotensin converting enzyme inhibitor enalapril in patients with essential hypertension. J Hypertens. 1995;13:1343–51.
Zanchetti A, Omboni S. Comparison of candesartan versus enalapril in essential hypertension. Italian Candesartan Study Group. Am J Hypertens. 2001;14:129–34.
Arima H, Chalmers J, Woodward M, Anderson C, Rodgers A, Davis S, et al. Lower target blood pressures are safe and effective for the prevention of recurrent stroke: the PROGRESS trial. J Hypertens. 2006;24:1201–8.
Barnett AH, Bain SC, Bouter P, Karlberg B, Madsbad S, Jervell J, et al. Angiotensin-receptor blockade versus converting-enzyme inhibition in type 2 diabetes and nephropathy. N Engl J Med. 2004;351:1952–61.
Foulquier S, Böhm M, Schmieder R, Sleight P, Teo K, Yusuf S, et al. Impact of telmisartan on cardiovascular outcome in hypertensive patients at high risk: a Telmisartan Randomised AssessmeNt Study in ACE iNtolerant subjects with cardiovascular Disease subanalysis. J Hypertens. 2014;32:1334–41.
Gustafsson F, Torp-Pedersen C, Kober L, Hildebrandt P, Køber L, Hildebrandt P. Effect of angiotensin converting enzyme inhibition after acute myocardial infarction in patients with arterial hypertension. TRACE Study Group, Trandolapril Cardiac Event. J Hypertens. 1997;15:793–8.
Kenchaiah S, Davis BR, Braunwald E, Rouleau JL, Dagenais GR, Sussex B, et al. Antecedent hypertension and the effect of captopril on the risk of adverse cardiovascular outcomes after acute myocardial infarction with left ventricular systolic dysfunction: insights from the Survival and Ventricular Enlargement Trial. Am Heart J. 2004;148:356–64.
Kostis JB. The effect of enalapril on mortal and morbid events in patients with hypertension and left ventricular dysfunction. Am J Hypertens. 1995;8:909–14.
Lithell H, Hansson L, Skoog I, Elmfeldt D, Hofman A, Olofsson B, et al. The Study on Cognition and Prognosis in the Elderly (SCOPE): principal results of a randomized double-blind intervention trial. J Hypertens. 2003;21:875–86.
Yusuf S, Teo KK, Pogue J, Dyal L, Copland I, Schumacher H, et al. Telmisartan, ramipril, or both in patients at high risk for vascular events. N Engl J Med. 2008;358:1547–59.
Kober L, Torp-Pedersen C, Carlsen JE, Bagger H, Eliasen P, Lyngborg K, et al. A clinical trial of the angiotensin-converting-enzyme inhibitor trandolapril in patients with left ventricular dysfunction after myocardial infarction. Trandolapril Cardiac Evaluation (TRACE) Study Group. N Engl J Med. 1995;333:1670–6.
Yusuf S, Pitt B, Davis CE, Hood WB, Cohn JN. Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure. N Engl J Med. 1991;325:293–302.
Pfeffer MA, Braunwald E, Moye LA, Basta L, Brown EJ Jr, et al. Effect of captopril on mortality and morbidity in patients with left ventricular dysfunction after myocardial infarction. Results of the survival and ventricular enlargement trial. The SAVE Investigators. N Engl J Med. 1992;327:669–77.
Thomopoulos C, Parati G, Zanchetti A. Effects of blood pressure-lowering on outcome incidence in hypertension: 5. Head-to-head comparisons of various classes of antihypertensive drugs - overview and meta-analyses. J Hypertens. 2015;33:1321–41.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
An interim analysis of the results has been presented during the European Society of Hypertension 2018 Congress in Barcelona, Spain.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Dimou, C., Antza, C., Akrivos, E. et al. A systematic review and network meta-analysis of the comparative efficacy of angiotensin-converting enzyme inhibitors and angiotensin receptor blockers in hypertension. J Hum Hypertens 33, 188–201 (2019). https://doi.org/10.1038/s41371-018-0138-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41371-018-0138-y