Abstract
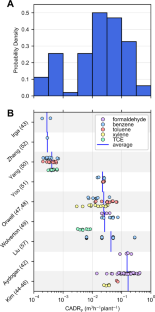
Potted plants have demonstrated abilities to remove airborne volatile organic compounds (VOC) in small, sealed chambers over timescales of many hours or days. Claims have subsequently been made suggesting that potted plants may reduce indoor VOC concentrations. These potted plant chamber studies reported outcomes using various metrics, often not directly applicable to contextualizing plants’ impacts on indoor VOC loads. To assess potential impacts, 12 published studies of chamber experiments were reviewed, and 196 experimental results were translated into clean air delivery rates (CADR, m3/h), which is an air cleaner metric that can be normalized by volume to parameterize first-order loss indoors. The distribution of single-plant CADR spanned orders of magnitude, with a median of 0.023 m3/h, necessitating the placement of 10–1000 plants/m2 of a building’s floor space for the combined VOC-removing ability by potted plants to achieve the same removal rate that outdoor-to-indoor air exchange already provides in typical buildings (~1 h−1). Future experiments should shift the focus from potted plants’ (in)abilities to passively clean indoor air, and instead investigate VOC uptake mechanisms, alternative biofiltration technologies, biophilic productivity and well-being benefits, or negative impacts of other plant-sourced emissions, which must be assessed by rigorous field work accounting for important indoor processes.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 6 print issues and online access
$259.00 per year
only $43.17 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Klepeis NE, Nelson WC, Ott WR, Robinson JP, Tsang AM, Switzer P, et al. The National Human Activity Pattern Survey (NHAPS): a resource for assessing exposure to environmental pollutants. J Exposure Sci Environ Epidemiol. 2001;11:231–52.
Weschler CJ. Ozone’s impact on public health: contributions from indoor exposures to ozone and products of ozone-initiated chemistry. Environ Health Perspect. 2006;114:1489–96.
Wallace L. Indoor particles: a review. J Air Waste Manag Assoc. 1996;46:98–126.
Wallace L. Indoor sources of ultrafine and accumulation mode particles: size distributions, size-resolved concentrations, and source strengths. Aerosol Sci Technol. 2006;40:348–60.
Weschler CJ, Shields HC. Production of the hydroxyl radical in indoor air. Environ Sci Technol. 1996;30:3250–8.
Weschler CJ, Nazaroff WW. Semivolatile organic compounds in indoor environments. Atmos Environ. 2008;42:9018–40.
Brown SK, Sim MR, Abramson MJ, Gray CN. Concentrations of volatile organic compounds in indoor air—a review. Indoor Air. 1994;4:123–34.
Morawska L, Afshari A, Bae GN, Buonanno G, Chao CYH, Hänninen O, et al. Indoor aerosols: from personal exposure to risk assessment. Indoor Air. 2013;23:462–87.
Johnson AM, Waring MS, DeCarlo PF. Real-time transformation of outdoor aerosol components upon transport indoors measured with aerosol mass spectrometry. Indoor Air. 2017;27:230–40.
Avery AM, Waring MS, DeCarlo PF. Seasonal variation in aerosol composition and concentration upon transport from the outdoor to indoor environment. Environ Sci: Process Impacts. 2019;21:528–47.
Uhde E, Salthammer T. Impact of reaction products from building materials and furnishings on indoor air quality—a review of recent advances in indoor chemistry. Atmos Environ. 2007;41:3111–28.
Nazaroff WW, Weschler CJ. Cleaning products and air fresheners: exposure to primary and secondary air pollutants. Atmos Environ. 2004;38:2841–65.
Huang Y, Ho SSH, Ho KF, Lee SC, Yu JZ, Louie PKK. Characteristics and health impacts of VOCs and carbonyls associated with residential cooking activities in Hong Kong. J Hazard Mater. 2011;186:344–51.
Brinke JT, Selvin S, Hodgson AT, Fisk WJ, Mendell MJ, Koshland CP, et al. Development of new volatile organic compound (VOC) exposure metrics and their relationship to “sick building syndrome” symptoms. Indoor Air 1998;8:140–52.
Jones AP. Indoor air quality and health. Atmos Environ. 1999;33:4535–64.
Wallace LA. Human exposure to volatile organic pollutants: implications for indoor air studies. Annu Rev Energy Environ. 2001;26:269–301.
Wieslander G, Norbäck D, Edling C. Airway symptoms among house painters in relation to exposure to volatile organic compounds (VOCs)—a longitudinal study. Ann Occup Hyg. 1997;41:155–66.
Yu C, Crump D. A review of the emission of VOCs from polymeric materials used in buildings. Build Environ. 1998;33:357–74.
Waring MS. Secondary organic aerosol in residences: predicting its fraction of fine particle mass and determinants of formation strength. Indoor Air. 2014;24:376–89.
Youssefi S, Waring MS. Predicting secondary organic aerosol formation from terpenoid ozonolysis with varying yields in indoor environments. Indoor Air. 2012;22:415–26.
Waring MS, Wells JR. Volatile organic compound conversion by ozone, hydroxyl radicals, and nitrate radicals in residential indoor air: Magnitudes and impacts of oxidant sources. Atmos Environ (1994). 2015;106:382–91.
Cummings BE, Waring MS. Predicting the importance of oxidative aging on indoor organic aerosol concentrations using the two-dimensional volatility basis set (2D-VBS). Indoor Air 2019;29:616–29.
Youssefi S, Waring MS. Indoor transient SOA formation from ozone+α-pinene reactions: Impacts of air exchange and initial product concentrations, and comparison to limonene ozonolysis. Atmos Environ. 2015;112:106–15.
Youssefi S, Waring MS. Transient secondary organic aerosol formation from limonene ozonolysis in indoor environments: impacts of air exchange rates and initial concentration ratios. Environ Sci Technol. 2014;48:7899–908.
Yang Y, Waring MS. Secondary organic aerosol formation initiated by α-terpineol ozonolysis in indoor air. Indoor Air. 2016;26:939–52.
Rohr AC. The health significance of gas- and particle-phase terpene oxidation products: a review. Environ Int. 2013;60:145–62.
Hallquist M, Wenger JC, Baltensperger U, Rudich Y, Simpson D, Claeys M, et al. The formation, properties and impact of secondary organic aerosol: current and emerging issues. Atmos Chem Phys. 2009;9:5155–236.
Lin Y-H, Arashiro M, Clapp PW, Cui T, Sexton KG, Vizuete W, et al. Gene expression profiling in human lung cells exposed to isoprene-derived secondary organic aerosol. Environ Sci Technol. 2017;51:8166–75.
Wargocki P, Sundell J, Bischof W, Brundrett G, Fanger PO, Gyntelberg F, et al. Ventilation and health in non-industrial indoor environments: report from a European multidisciplinary scientific consensus meeting (EUROVEN). Indoor Air. 2002;12:113–28.
Mendell MJ, Eliseeva EA, Davies MM, Spears M, Lobscheid A, Fisk WJ, et al. Association of classroom ventilation with reduced illness absence: a prospective study in California elementary schools. Indoor Air. 2013;23:515–28.
Wargocki P, Wyon DP, Fanger PO. The performance and subjective responses of call-center operators with new and used supply air filters at two outdoor air supply rates. Indoor Air. 2004;14 Suppl 8:7–16.
Haverinen‐Shaughnessy U, Moschandreas DJ, Shaughnessy RJ. Association between substandard classroom ventilation rates and students’ academic achievement. Indoor Air 2011;21:121–31.
Carrer P, Wargocki P, Fanetti A, Bischof W, De Oliveira Fernandes E, Hartmann T, et al. What does the scientific literature tell us about the ventilation–health relationship in public and residential buildings? Build Environ. 2015;94:273–86.
Fisk WJ, Mirer AG, Mendell MJ. Quantification of the association of ventilation rates with sick building syndrome symptoms. Berkeley, CA, USA: Lawrence Berkeley National Lab. (LBNL); 2009. Report No.: LBNL-2035E. https://www.osti.gov/biblio/962711
Rackes A, Ben‐David T, Waring MS. Outcome-based ventilation: a framework for assessing performance, health, and energy impacts to inform office building ventilation decisions. Indoor Air. 2018;28:585–603.
Quang TN, He C, Morawska L, Knibbs LD. Influence of ventilation and filtration on indoor particle concentrations in urban office buildings. Atmos Environ. 2013;79:41–52.
Weschler CJ. Ozone in indoor environments: concentration and chemistry. Indoor Air. 2000;10:269–88.
Ben-David T, Wang S, Rackes A, Waring MS. Measuring the efficacy of HVAC particle filtration over a range of ventilation rates in an office building. Build Environ. 2018;144:648–56.
Benne K, Griffith B, Long N, Torcellini P, Crawley D, Logee T. Assessment of the energy impacts of outside air in the commercial sector. 2009. Report No.: NREL/TP-550-41955, 951796. http://www.osti.gov/servlets/purl/951796-l2ErYY/
Rackes A, Waring MS. Alternative ventilation strategies in U.S. offices: Comprehensive assessment and sensitivity analysis of energy saving potential. Build Environ. 2017;116:30–44.
Ben-David T, Rackes A, Waring MS. Alternative ventilation strategies in U.S. offices: Saving energy while enhancing work performance, reducing absenteeism, and considering outdoor pollutant exposure tradeoffs. Build Environ. 2017;116:140–57.
Aydogan A, Montoya LD. Formaldehyde removal by common indoor plant species and various growing media. Atmos Environ. 2011;45:2675–82.
Irga PJ, Torpy FR, Burchett MD. Can hydroculture be used to enhance the performance of indoor plants for the removal of air pollutants? Atmos Environ. 2013;77:267–71.
Kim KJ, Kil MJ, Song JS, Yoo EH, Son K-C, Kays SJ. Efficiency of volatile formaldehyde removal by indoor plants: contribution of aerial plant parts versus the root zone. J Am Soc Hort Sci. 2008;133:521–6.
Kim KJ, Jeong MI, Lee DW, Song JS, Kim HD, Yoo EH, et al. Variation in formaldehyde removal efficiency among indoor plant species. HortScience 2010;45:1489–95.
Kim KJ, Kim HJ, Khalekuzzaman M, Yoo EH, Jung HH, Jang HS. Removal ratio of gaseous toluene and xylene transported from air to root zone via the stem by indoor plants. Environ Sci Pollut Res. 2016;23:6149–58.
Orwell RL, Wood RL, Tarran J, Torpy F, Burchett MD. Removal of benzene by the indoor plant/substrate microcosm and implications for air quality. Water Air Soil Pollut. 2004;157:193–207.
Orwell RL, Wood RA, Burchett MD, Tarran J, Torpy F. The potted-plant microcosm substantially reduces indoor air VOC pollution: II. Laboratory study. Water Air Soil Pollut. 2006;177:59–80.
Wolverton BC, Johnson A, Bounds K. Interior landscape plants for indoor air pollution abatement. 1989. Report No.: NASA-TM-101766. https://ntrs.nasa.gov/search.jsp?R=19930073077
Yang DS, Pennisi SV, Son K-C, Kays SJ. Screening indoor plants for volatile organic pollutant removal efficiency. HortScience 2009;44:1377–81.
Yoo MH, Kwon YJ, Son K-C, Kays SJ. Efficacy of indoor plants for the removal of single and mixed volatile organic pollutants and physiological effects of the volatiles on the plants. J Am Soc Horticultural Sci 2006;131:452–8.
Zhang L, Routsong R, Strand SE. Greatly enhanced removal of volatile organic carcinogens by a genetically modified houseplant, pothos Ivy (Epipremnum aureum) expressing the mammalian cytochrome P450 2e1 gene. Environ Sci Technol. 2019;53:325–31.
Rackes A, Waring MS. Do time-averaged, whole-building, effective volatile organic compound (VOC) emissions depend on the air exchange rate? A statistical analysis of trends for 46 VOCs in U.S. offices. Indoor Air 2016;26:642–59.
Weisel CP, Zhang J, Turpin BJ, Morandi MT, Colome S, Stock TH, et al. Relationship of indoor, outdoor and personal air (RIOPA) study: study design, methods and quality assurance/control results. J Expo Sci Environ Epidemiol. 2005;15:123–37.
Turpin BJ, Weisel CP, Morandi M, Colome S, Stock T, Eisenreich S, et al. Relationships of indoor, outdoor, and personal air (RIOPA): part II. Analyses of concentrations of particulate matter species. Res Rep Health Eff Inst. 2007;130 Pt 2:1–77. discussion 79–92.
Xu Z, Wang L, Hou H. Formaldehyde removal by potted plant–soil systems. J Hazard Mater. 2011;192:314–8.
Liu Y-J, Mu Y-J, Zhu Y-G, Ding H, Crystal Arens N. Which ornamental plant species effectively remove benzene from indoor air? Atmos Environ. 2007;41:650–4.
Cruz MD, Müller R, Svensmark B, Pedersen JS, Christensen JH. Assessment of volatile organic compound removal by indoor plants—a novel experimental setup. Environ Sci Pollut Res. 2014;21:7838–46.
Hörmann V, Brenske K-R, Ulrichs C. Suitability of test chambers for analyzing air pollutant removal by plants and assessing potential indoor air purification. Water Air Soil Pollut. 2017;228:402.
Girman J, Phillips T, Levin H. Critical review: how well do house plants perform as indoor air cleaners? 2009;5.
Dingle P, Tapsell P, Hu S. Reducing formaldehyde exposure in office environments using plants. Bull Environ Contam Toxicol. 2000;64:302–8.
Wood RA, Burchett MD, Alquezar R, Orwell RL, Tarran J, Torpy F. The potted-plant microcosm substantially reduces indoor air VOC pollution: I. Office field-study. Water Air Soil Pollut. 2006;175:163–80.
Levin H. Can house plants solve IAQ problems?. Indoor Air Bull. 1992;2:1–7.
Wood RA, Orwell RL, Tarran J, Torpy F, Burchett M. Potted-plant/growth media interactions and capacities for removal of volatiles from indoor air. J Horticult Sci Biotechnol. 2002;77:120–9.
Waring MS, Siegel JA, Corsi RL. Ultrafine particle removal and generation by portable air cleaners. Atmos Environ. 2008;42:5003–14.
Kim H-J, Han B, Kim Y-J, Yoon Y-H, Oda T. Efficient test method for evaluating gas removal performance of room air cleaners using FTIR measurement and CADR calculation. Build Environ. 2012;47:385–93.
Chen W, Zhang J, Zhang ZB. Performance of air cleaners for removing multi-volatile organic compounds in indoor air. ASHRAE Trans. 2005;111:1101–14.
Russell JA, Hu Y, Chau L, Pauliushchyk M, Anastopoulos I, Anandan S, et al. Indoor-biofilter growth and exposure to airborne chemicals drive similar changes in plant root bacterial communities. Appl Environ Microbiol. 2014;80:4805–13.
Darlington A, Chan M, Malloch D, Pilger C, Dixon MA. The biofiltration of indoor air: implications for air quality. Indoor Air 2000;10:39–46.
Darlington AB, Dat JF, Dixon MA. The biofiltration of indoor air: air flux and temperature influences the removal of toluene, ethylbenzene, and xylene. Environ Sci Technol. 2001;35:240–6.
Alraddadi O, Leuner H, Boor B, Rajkhowa B, Hutzel W, Dana M. Air cleaning performance of a biowall for residential applications. International High Performance Buildings Conference. 2016. https://docs.lib.purdue.edu/ihpbc/185
Wang Z, Zhang JS. Characterization and performance evaluation of a full-scale activated carbon-based dynamic botanical air filtration system for improving indoor air quality. Build Environ. 2011;46:758–68.
Soreanu G, Dixon M, Darlington A. Botanical biofiltration of indoor gaseous pollutants—a mini-review. Chem Eng J. 2013;229:585–94.
Mikkonen A, Li T, Vesala M, Saarenheimo J, Ahonen V, Kärenlampi S, et al. Biofiltration of airborne VOCs with green wall systems—microbial and chemical dynamics. Indoor Air. https://onlinelibrary.wiley.com/doi/abs/10.1111/ina.12473. 2018.
Bringslimark T, Hartig T, Patil GG. The psychological benefits of indoor plants: A critical review of the experimental literature. J Environ Psychol. 2009;29:422–33.
Peñuelas J, Llusià J. Plant VOC emissions: making use of the unavoidable. Trends Ecol Evolution. 2004;19:402–4.
Holopainen JK, Gershenzon J. Multiple stress factors and the emission of plant VOCs. Trends Plant Sci. 2010;15:176–84.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Cummings, B.E., Waring, M.S. Potted plants do not improve indoor air quality: a review and analysis of reported VOC removal efficiencies. J Expo Sci Environ Epidemiol 30, 253–261 (2020). https://doi.org/10.1038/s41370-019-0175-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41370-019-0175-9
Keywords
This article is cited by
-
Phytoremediation for the indoor environment: a state-of-the-art review
Reviews in Environmental Science and Bio/Technology (2023)
-
Effective mitigation strategies for reducing workers’ exposure to formaldehyde: a systematic review
Air Quality, Atmosphere & Health (2023)
-
Potted plants can remove the pollutant nitrogen dioxide indoors
Air Quality, Atmosphere & Health (2022)
-
Effects of airflow rate and plant species on formaldehyde removal by active green walls
Environmental Science and Pollution Research (2022)



