Abstract
Background
4−20% of people report using cannabis during pregnancy, thereby it is essential to assess the associated risks. There is some evidence that prenatal cannabis exposure (PCE) may be associated with increased risk for developing of obesity and diabetes later in life, however this has not been well explored under controlled conditions. The aim of this study was to use a translational THC vapor model in rodents to characterize the effects of PCE on adiposity, glucose metabolism, and feeding patterns in adulthood, with focus on potential sex differences.
Methods
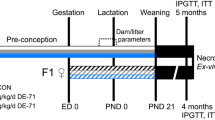
Pregnant Sprague Dawley rats were exposed to vaporized THC (100 mg/ml) or control (polyethylene glycol vehicle) across the entire gestational period. Adult offspring from PCE (n = 24) or control (n = 24) litters were subjected to measures of adiposity, glucose metabolism and feeding behavior. Rats were then placed onto special diets (60% high-fat diet [HFD] or control 10% low fat diet [LFD]) for 4-months, then re-subjected to adiposity, glucose metabolism and feeding behavior measurements.
Results
PCE did not influence maternal weight or food consumption but was associated with transient decreased pup weight. PCE did not initially influence bodyweight or adiposity, but PCE did significantly reduce the rate of bodyweight gain when on HFD/LFD, regardless of which diet. Further, PCE had complex effects on glucose metabolism and feeding behavior that were both sex and diet dependent. No effects of PCE were found on plasma leptin or insulin, or white adipose tissue mass.
Conclusions
PCE may not promote obesity development but may increase risk for diabetes and abnormal eating habits under certain biological and environmental conditions. Overall, this data enhances current understanding of the potential impacts of PCE.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The datasets generated and/or analysed in this manuscript are available from the corresponding author on reasonable request.
References
Goodman S, Wadsworth E, Leos-Toro C, Hammond D, Team ICPS. Prevalence and forms of cannabis use in legal vs. illegal recreational cannabis markets. Int J Drug Policy. 2020;76:102658.
Ko JY, Farr SL, Tong VT, Creanga AA, Callaghan WM. Prevalence and patterns of marijuana use among pregnant and nonpregnant women of reproductive age. Am J Obstet Gynecol. 2015;213:201.e1–201.e10.
Young-Wolff KC, Tucker L-Y, Alexeeff S, Armstrong MA, Conway A, Weisner C, et al. Trends in self-reported and biochemically tested marijuana use among pregnant females in California from 2009-2016. JAMA. 2017;318:2490–1.
Vanstone M, Taneja S, Popoola A, Panday J, Greyson D, Lennox R, et al. Reasons for cannabis use during pregnancy and lactation: a qualitative study. CMAJ. 2021;193:E1906–14.
Dickson B, Mansfield C, Guiahi M, Allshouse AA, Borgelt LM, Sheeder J, et al. Recommendations from cannabis dispensaries about first-trimester cannabis use. Obstet Gynecol. 2018;131:1031.
ElSohly MA, Chandra S, Radwan M, Majumdar CG, Church JC. A comprehensive review of cannabis potency in the United States in the last decade. Biol Psychiatry: Cogn Neurosci Neuroimaging. 2021;6:603–6.
Baglot SL, Hume C, Petrie GN, Aukema RJ, Lightfoot SH, Grace LM, et al. Pharmacokinetics and central accumulation of delta-9-tetrahydrocannabinol (THC) and its bioactive metabolites are influenced by route of administration and sex in rats. Sci Rep. 2021;11:23990.
Grotenhermen F. Pharmacokinetics and pharmacodynamics of cannabinoids. Clin Pharmacokinetics. 2003;42:327–60.
Benevenuto SG, Domenico MD, Martins MAG, Costa NS, de Souza ARL, Costa JL, et al. Recreational use of marijuana during pregnancy and negative gestational and fetal outcomes: an experimental study in mice. Toxicology. 2017;376:94–101.
Gillies R, Lee K, Vanin S, Laviolette SR, Holloway AC, Arany E, et al. Maternal exposure to Δ9-tetrahydrocannabinol impairs female offspring glucose homeostasis and endocrine pancreatic development in the rat. Reprod Toxicol. 2020;94:84–91.
Hayatbakhsh MR, Flenady VJ, Gibbons KS, Kingsbury AM, Hurrion E, Mamun AA, et al. Birth outcomes associated with cannabis use before and during pregnancy. Pediatr Res. 2012;71:215–9.
McLemore GL, Richardson KA. Data from three prospective longitudinal human cohorts of prenatal marijuana exposure and offspring outcomes from the fetal period through young adulthood. Data Brief. 2016;9:753–7.
Moore BF, Sauder KA, Shapiro AL, Crume T, Kinney GL, Dabelea D. Fetal exposure to cannabis and childhood metabolic outcomes: the healthy start study. J Clin Endocrinol Metab. 2022;107:e2862–9.
Natale BV, Gustin KN, Lee K, Holloway AC, Laviolette SR, Natale DR, et al. Δ9-tetrahydrocannabinol exposure during rat pregnancy leads to symmetrical fetal growth restriction and labyrinth-specific vascular defects in the placenta. Sci Rep. 2020;10:544.
Paul SE, Hatoum AS, Fine JD, Johnson EC, Hansen I, Karcher NR, et al. Associations between prenatal cannabis exposure and childhood outcomes: results from the ABCD study. JAMA Psychiatry. 2021;78:64–76.
Rodriguez CE, Sheeder J, Allshouse AA, Scott S, Wymore E, Hopfer C, et al. Marijuana use in young mothers and adverse pregnancy outcomes: a retrospective cohort study. BJOG: Int J Obstet Gynaecol. 2019;126:1491–7.
Weimar HV, Wright HR, Warrick CR, Brown AM, Lugo JM, Freels TG, et al. Long-term effects of maternal cannabis vapor exposure on emotional reactivity, social behavior, and behavioral flexibility in offspring. Neuropharmacology. 2020;179:108288.
Campolongo P, Trezza V, Cassano T, Gaetani S, Morgese MG, Ubaldi M, et al. PRECLINICAL STUDY: perinatal exposure to delta‐9‐tetrahydrocannabinol causes enduring cognitive deficits associated with alteration of cortical gene expression and neurotransmission in rats. Addiction Biol. 2007;12:485–95.
Corsi DJ, Donelle J, Sucha E, Hawken S, Hsu H, El-Chaâr D, et al. Maternal cannabis use in pregnancy and child neurodevelopmental outcomes. Nat Med. 2020;26:1536–40.
Porath-Waller AJ. (May 2022 Update). Clearing the smoke on cannabis: maternal cannabis use during pregnancy: Canadian Centre on Substance Abuse. 2009. https://www.ccsa.ca/clearing-smoke-cannabis-cannabis-use-during-pregnancy-and-breastfeeding
Trezza V, Campolongo P, Cassano T, Macheda T, Dipasquale P, Carratù MR, et al. Effects of perinatal exposure to delta-9-tetrahydrocannabinol on the emotional reactivity of the offspring: a longitudinal behavioral study in Wistar rats. Psychopharmacology. 2008;198:529–37.
Day NL, Leech SL, Goldschmidt L. The effects of prenatal marijuana exposure on delinquent behaviors are mediated by measures of neurocognitive functioning. Neurotoxicol Teratol. 2011;33:129–36.
Sonon K, Richardson GA, Cornelius J, Kim KH, Day NL. Developmental pathways from prenatal marijuana exposure to Cannabis Use Disorder in young adulthood. Neurotoxicol Teratol. 2016;58:46–52.
Spano MS, Ellgren M, Wang X, Hurd YL. Prenatal cannabis exposure increases heroin seeking with allostatic changes in limbic enkephalin systems in adulthood. Biol Psychiatry. 2007;61:554–63.
Roncero C, Valriberas-Herrero I, Mezzatesta-Gava M, Villegas JL, Aguilar L, Grau-López L.Cannabis use during pregnancy and its relationship with fetal developmental outcomes and psychiatric disorders. A systematic review.Reprod Health. 2020;17:1–9.
Baglot SL, VanRyzin JW, Marquardt AE, Aukema RJ, Petrie GN, Hume C, et al. Maternal‐fetal transmission of delta‐9‐tetrahydrocannabinol (THC) and its metabolites following inhalation and injection exposure during pregnancy in rats. J Neurosci Res. 2022;100:713–30.
Hložek T, Uttl L, Kadeřábek L, Balíková M, Lhotková E, Horsley RR, et al. Pharmacokinetic and behavioural profile of THC, CBD, and THC+ CBD combination after pulmonary, oral, and subcutaneous administration in rats and confirmation of conversion in vivo of CBD to THC. Eur Neuropsychopharmacol. 2017;27:1223–37.
Huestis MA. Human cannabinoid pharmacokinetics. Chem Biodivers. 2007;4:1770.
Nguyen JD, Aarde SM, Vandewater SA, Grant Y, Stouffer DG, Parsons LH, et al. Inhaled delivery of Δ9-tetrahydrocannabinol (THC) to rats by e-cigarette vapor technology. Neuropharmacology. 2016;109:112–20.
Torrens A, Vozella V, Huff H, McNeil B, Ahmed F, Ghidini A, et al. Comparative pharmacokinetics of Δ9-tetrahydrocannabinol in adolescent and adult male mice. J Pharmacol Exp Therapeutics. 2020;374:151–60.
National Academies of Sciences, Engineering, and Medicine. The Health Effects of Cannabis and Cannabinoids: The Current State of Evidence and Recommendations for Research. Washington (DC): National Academies Press (US); 2017. https://www.ncbi.nlm.nih.gov/books/NBK423845/, https://doi.org/10.17226/24625
Heindel JJ, Vandenberg LN. Developmental origins of health and disease: a paradigm for understanding disease etiology and prevention. Curr Opin Pediatr. 2015;27:248.
Cajachagua‐Torres KN, El Marroun H, Reiss IK, Santos S, Jaddoe VW. Foetal tobacco and cannabis exposure, body fat and cardio‐metabolic health in childhood. Pediatr Obes. 2022;17:e12863.
Lee K, Hardy DB. Metabolic consequences of gestational cannabinoid exposure. Int J Mol Sci. 2021;22:9528.
Oke SL, Lee K, Papp R, Laviolette SR, Hardy DB. In utero exposure to δ9-tetrahydrocannabinol leads to postnatal catch-up growth and dysmetabolism in the adult rat liver. Int J Mol Sci. 2021;22:7502.
Ellis RJ, Bara A, Vargas CA, Frick AL, Loh E, Landry J, et al. Prenatal Δ9-tetrahydrocannabinol exposure in males leads to motivational disturbances related to striatal epigenetic dysregulation. Biol Psychiatry. 2022;92:127–38.
Konkle A, Sreter K, Baker S, Bielajew C. Chronic paroxetine infusion influences macronutrient selection in male Sprague–Dawley rats. Pharmacol Biochem Behav. 2003;74:883–90.
Shor-Posner G, Brennan G, Ian C, Jasaitis R, Madhu K, Leibowitz SF. Meal patterns of macronutrient intake in rats with particular dietary preferences. Am J Physiol-Regulatory, Integr Comp Physiol. 1994;266:R1395–R402.
Sticht MA, Lau DJ, Keenan CM, Cavin JB, Morena M, Vemuri VK, et al. Endocannabinoid regulation of homeostatic feeding and stress‐induced alterations in food intake in male rats. Br J Pharmacol. 2019;176:1524–40.
Vagena E, Ryu JK, Baeza-Raja B, Walsh NM, Syme C, Day JP, et al. A high-fat diet promotes depression-like behavior in mice by suppressing hypothalamic PKA signaling. Transl psychiatry. 2019;9:141.
Johnson P, Hirsch J. Cellularity of adipose depots in six strains of genetically obese mice. J Lipid Res. 1972;13:2–11.
Fearby N, Penman S, Thanos P. Effects of Δ9-Tetrahydrocannibinol (THC) on obesity at different stages of life: a literature review. Int J Environ Res Public Health. 2022;19:3174.
Chang X, Bian Y, He Q, Yao J, Zhu J, Wu J, et al. Suppression of STAT3 signaling by Δ9-tetrahydrocannabinol (THC) induces trophoblast dysfunction. Cell Physiol Biochem. 2017;42:537–50.
Wu C-S, Jew CP, Lu H-C. Lasting impacts of prenatal cannabis exposure and the role of endogenous cannabinoids in the developing brain. Future Neurol. 2011;6:459–80.
Coppey LJ, Shevalye H, Obrosov A, Davidson EP, Yorek MA. Determination of peripheral neuropathy in high‐fat diet fed low‐dose streptozotocin‐treated female C57Bl/6J mice and Sprague–Dawley rats. J Diabetes Investig. 2018;9:1033–40.
Li G, Zhang Y, Wilsey J, Scarpace P. Unabated anorexic and enhanced thermogenic responses to melanotan II in diet-induced obese rats despite reduced melanocortin 3 and 4 receptor expression. J Endocrinol. 2004;182:123–32.
Cluny NL, Keenan CM, Reimer RA, Le Foll B, Sharkey KA. Prevention of diet-induced obesity effects on body weight and gut microbiota in mice treated chronically with Δ9-tetrahydrocannabinol. PloS one. 2015;10:e0144270.
Lin L, Jung K-M, Lee H-L, Le J, Colleluori G, Wood C, et al. Adolescent exposure to low-dose THC disrupts energy balance and adipose organ homeostasis in adulthood. Cell Metab. 2023;35:1227–1241.e7.
Asadi F, Andrade JAF, Gillies R, Lee K, Dhanvantari S, Hardy DB, et al. Sex-dependent effect of in-utero exposure to Δ9-Tetrahydrocannabinol on Glucagon and Stathmin-2 in adult rat offspring. Can J Diabetes. 2022;46:851–62.
Didier L, Yerby B, Deacon R, Gao J. Diet-induced modulation of mitochondrial activity in rat muscle. Am J Physiol-Endocrinol Metab. 2007;293:E1169–77.
Bermúdez-Silva F, Suárez J, Baixeras E, Cobo N, Bautista D, Cuesta-Muñoz A, et al. Presence of functional cannabinoid receptors in human endocrine pancreas. Diabetologia. 2008;51:476–87.
Kim J, Lee KJ, Kim JS, Rho JG, Shin JJ, Song WK, et al. Cannabinoids regulate Bcl-2 and cyclin D2 expression in pancreatic β cells. PLoS One. 2016;11:e0150981.
Malenczyk K, Keimpema E, Piscitelli F, Calvigioni D, Björklund P, Mackie K, et al. Fetal endocannabinoids orchestrate the organization of pancreatic islet microarchitecture. Proc Natl Acad Sci. 2015;112:E6185–94.
Kim W, Doyle ME, Liu Z, Lao Q, Shin Y-K, Carlson OD, et al. Cannabinoids inhibit insulin receptor signaling in pancreatic β-cells. Diabetes. 2011;60:1198–1209.
Guillaumin MC, Peleg-Raibstein D. Maternal over-and malnutrition and increased risk for addictive and eating disorders in the offspring. Nutrients. 2023;15:1095.
Sominsky L, Spencer SJ. Eating behavior and stress: a pathway to obesity. Front Psychol. 2014;5:434.
Primo MJ, Fonseca-Rodrigues D, Almeida A, Teixeira PM, Pinto-Ribeiro F. Sucrose preference test: a systematic review of protocols for the assessment of anhedonia in rodents. Eur Neuropsychopharmacol. 2023;77:80–92.
Richardson KA, Hester AK, McLemore GL. Prenatal cannabis exposure-The “first hit” to the endocannabinoid system. Neurotoxicol Teratol. 2016;58:5–14.
Jeong JY, Lee DH, Kang SS. Effects of chronic restraint stress on body weight, food intake, and hypothalamic gene expressions in mice. Endocrinol Metab. 2013;28:288–96.
Peltier MR, Roberts W, Verplaetse TL, Burke C, Zakiniaeiz Y, Moore K, et al. Licit and illicit drug use across trimesters in pregnant women endorsing past-year substance use: results from National Survey on Drug Use and Health (2009–2019). Arch Women’s Ment health. 2022;25:819–27.
Palmer AK, Jensen MD. Metabolic changes in aging humans: current evidence and therapeutic strategies. J Clin Investig. 2022;132:e158451.
Wali JA, Ni D, Facey HJ, Dodgson T, Pulpitel TJ, Senior AM, et al. Determining the metabolic effects of dietary fat, sugars and fat-sugar interaction using nutritional geometry in a dietary challenge study with male mice. Nat Commun. 2023;14:4409.
Moore CF, Stiltner JW, Davis CM, Weerts EM. Translational models of cannabinoid vapor exposure in laboratory animals. Behav Pharmacol. 2022;33:63–89.
Acknowledgements
We would like to acknowledge that Indigenous peoples are the original and current caretakers of the land we live, work, learn, and play on. Specifically, our research was conducted on the traditional territories of the people of the Treaty 7 region in Southern Alberta, which includes the Blackfoot Confederacy (comprising the Siksika, Piikani, and Kainai First Nations), as well as the Tsuut’ina First Nation, and the Stoney Nakoda (including the Chiniki, Bearspaw, and Wesley First Nations). The City of Calgary is also home to Métis Nation of Alberta (District 5 and 6). As an academic community, we must recognize that their land was taken through coercive and violent acts, and we must support their authority and rights over this stolen land. We must acknowledge our responsibility to establish and maintain relationships with Indigenous peoples and we must include their voices in our teaching and research. We would also like to thank Dr Lauren Seabrook for advice and assistance with data analysis and glucose tolerance testing; Dr Georgia Balsevich for experimental design advice; and finally Andrei (Sabin) Nastase, Dr Robert Aukema and Dr Gavin Petrie for data collection help.
Funding
This research is funded by operating funds from the Canadian Institutes of Health Research (CIHR) held by MNH. CH received salary support from a Hotchkiss Brain Institute (HBI) Harley Hotchkiss - Samuel Weiss Postdoctoral Fellowship, Alberta Children’s Hospital Research Institute (ACHRI) Postdoctoral Fellowship and Cumming School of Medicine Postdoctoral Fellowship. SLB received salary support from an ACHRI Graduate Scholarship, Brain Canada Rising Stars Trainee Award: CCIC Neuroscience Fellowship in Cannabis and Cannabinoid Research and Alberta Graduate Excellence Scholarship (AGES) – Indigenous. LJ received salary support from an Alberta Innovates Summer Research Studentship. SHML received salary support from a HBI Harley N Hotchkiss Graduate Scholarship in Neuroscience and AGES – Indigenous. JS received salary support from a CIHR Canada Graduate Scholarship – Master’s and HBI Graduate Scholarship in Neuroscience.
Author information
Authors and Affiliations
Contributions
Conceptualization and experimental design were carried out by CH, SLB and MNH. Breeding and PCE was carried out by SLB and SHML, with help from LJ and CH. Feeding behavior experiments were carried out by CH with help from LJ, SLB and JS. Glucose tolerance tests were carried out by CH and SLB with help from LJ, JS and SHML. WAT measurements, blood sampling and ELISA’s were carried out by CH and SLB. CH and SLB analyzed data and wrote the manuscript with help from LJ and MNH. All authors read and approved the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hume, C., Baglot, S.L., Javorcikova, L. et al. Effects of prenatal THC vapor exposure on body weight, glucose metabolism, and feeding behaviors in chow and high-fat diet fed rats. Int J Obes (2024). https://doi.org/10.1038/s41366-024-01512-8
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41366-024-01512-8