Abstract
Background
Asprosin (ASP) is a newly discovered adipokine secreted by white adipose tissue (WAT), which can regulate the homeostasis of glucose and lipid metabolism. However, it is not clear whether it can regulate the browning of WAT and mitophagy during the browning process. Accordingly, this study aims to investigate the effects and possible mechanisms of ASP on the browning of WAT and mitophagy in vivo and in vitro.
Methods
In in vivo experiments, some mouse models were used including adipose tissue ASP-specific deficiency (ASP–/–), high fat diet (HFD)-induced obesity and white adipose browning; in in vitro experiments, some cell models were also established and used, including ASP-deficient 3T3-L1 preadipocyte (ASP–/–) and CL-316243 (CL, 1 µM)-induced browning. Based on these models, the browning of WAT and mitophagy were evaluated by morphology, functionality and molecular markers.
Results
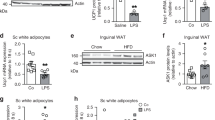
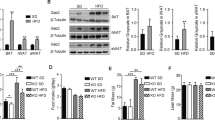
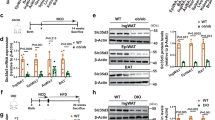
Our in vivo data show that adipose tissue-specific deletion of ASP contributes to weight loss in mice; supplementation of ASP inhibits the expressions of browning-related proteins including UCP1, PRDM16 and PGC1ɑ during the cold exposure-induced browning, and promotes the expressions of mitophagy-related proteins including PINK1 and Parkin under the conditions of whether normal diet (ND) or HFD. Similarly, our in vitro data also show that the deletion of ASP in 3T3-L1 cells significantly increases the expressions of the browning-related proteins and decreases the expressions of the mitophagy-related proteins.
Conclusions
These data demonstrate that ASP deletion can facilitate the browning and inhibit mitophagy in WAT. The findings will lay an experimental foundation for the development of new drugs targeting ASP and the clinical treatment of metabolic diseases related to obesity.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
All the data supporting the findings of the study were shown in this paper and are available upon reasonable request.
References
Okunogbe A, Nugent R, Spencer G, Powis J, Ralston J, Wilding J. Economic impacts of overweight and obesity: current and future estimates for 161 countries. BMJ Glob Health. 2022;7:e009773.
Rabiee A. Beige fat maintenance; toward a sustained metabolic health. Front Endocrinol (Lausanne). 2020;11:634.
Cohen P, Kajimura S. The cellular and functional complexity of thermogenic fat. Nat Rev Mol Cell Biol. 2021;22:393–409.
Zhu T, Chen X, Jiang SL. Progress and obstacles in transplantation of brown adipose tissue or engineered cells with thermogenic potential for metabolic benefits. Front Endocrinol (Lausanne). 2023;14:1191278.
Kurylowicz A, Puzianowska-Kuznicka M. Induction of adipose tissue browning as a strategy to combat obesity. Int J Mol Sci. 2020;21:6241
Xue SW, Lee D, Berry DC. Thermogenic adipose tissue in energy regulation and metabolic health. Front Endocrinol (Lausanne). 2023;14:1150059.
Saito M, Okamatsu-Ogura Y. Thermogenic brown fat in humans: implications in energy homeostasis, obesity and metabolic disorders. World J Mens Health. 2023;41:489–507.
Ferhat M, Funai K, Boudina S. Autophagy in adipose tissue physiology and pathophysiology. Antioxid Redox Signal. 2019;31:487–501.
Cho YK, Son Y, Saha A, Kim D, Choi C, Kim M, et al. STK3/STK4 signalling in adipocytes regulates mitophagy and energy expenditure. Nat Metab. 2021;3:428–41.
Goodall EA, Kraus F, Harper JW. Mechanisms underlying ubiquitin-driven selective mitochondrial and bacterial autophagy. Mol Cell. 2022;82:1501–13.
Ro SH, Jang Y, Bae J, Kim IM, Schaecher C, Shomo ZD. Autophagy in adipocyte browning: emerging drug target for intervention in obesity. Front Physiol. 2019;10:22.
Romere C, Duerrschmid C, Bournat J, Constable P, Jain M, Xia F, et al. Asprosin, a fasting-induced glucogenic protein hormone. Cell. 2016;165:566–79.
Farrag M, Ait Eldjoudi D, Gonzalez-Rodriguez M, Cordero-Barreal A, Ruiz-Fernandez C, Capuozzo M, et al. Asprosin in health and disease, a new glucose sensor with central and peripheral metabolic effects. Front Endocrinol (Lausanne). 2022;13:1101091.
Shabir K, Brown JE, Afzal I, Gharanei S, Weickert MO, Barber TM, et al. Asprosin, a novel pleiotropic adipokine implicated in fasting and obesity-related cardio-metabolic disease: Comprehensive review of preclinical and clinical evidence. Cytokine Growth Factor Rev. 2021;60:120–32.
Yin TT, Chen S, Zeng GH, Yuan WW, Lu YL, Zhang YN, et al. Angiogenesis-browning interplay mediated by asprosin-knockout contributes to weight loss in mice with obesity. Int J Mol Sci. 2022;23:16166
Lu YL, Yuan WW, Xiong XW, Huang QQ, Chen S, Yin TT, et al. Asprosin aggravates vascular endothelial dysfunction via disturbing mitochondrial dynamics in obesity models. Obesity (Silver Spring). 2023;31:732–43.
Huang QQ, Chen S, Xiong XW, Yin TT, Zhang YN, Zeng GH, et al. Asprosin exacerbates endothelium inflammation induced by hyperlipidemia through activating IKKbeta-NF-kappaBp65 pathway. Inflammation. 2023;46:623–38.
Zhang YN, Huang QQ, Xiong XW, Yin TT, Chen S, Yuan WW, et al. Acacetin alleviates energy metabolism disorder through promoting white fat browning mediated by AC-cAMP pathway. J Physiol Biochem. 2023;79:529–41.
Vamos A, Shaw A, Varga K, Csomos I, Mocsar G, Balajthy Z, et al. Mitophagy mediates the beige to white transition of human primary subcutaneous adipocytes ex vivo. Pharmaceuticals (Basel). 2022;15:363.
Lu XD, Altshuler-Keylin S, Wang Q, Chen Y, Henrique Sponton C, Ikeda K., et al. Mitophagy controls beige adipocyte maintenance through a Parkin-dependent and UCP1-independent mechanism. Sci Signal. 2018;11:eaap8526.
Lu XD. Maintaining mitochondria in beige adipose tissue. Adipocyte. 2019;8:77–82.
Camilleri M, Acosta A. Combination therapies for obesity. Metab Syndr Relat Disord. 2018;16:390–4.
Cheng L, Wang JK, Dai HY, Duan YH, An YC, Shi L, et al. Brown and beige adipose tissue: a novel therapeutic strategy for obesity and type 2 diabetes mellitus. Adipocyte. 2021;10:48–65.
Dodd GT, Decherf S, Loh K, Simonds SE, Wiede F, Balland E, et al. Leptin and insulin act on POMC neurons to promote the browning of white fat. Cell. 2015;160:88–104.
Kita S, Maeda N, Shimomura I. Interorgan communication by exosomes, adipose tissue, and adiponectin in metabolic syndrome. J Clin Invest. 2019;129:4041–9.
Ke F, Xue GH, Jiang XL, Li FF, Lai XY, Zhang MY, et al. Combination of asprosin and adiponectin as a novel marker for diagnosing non-alcoholic fatty liver disease. Cytokine. 2020;134:155184.
Zhang L, Chen C, Zhou N, Fu YM, Cheng XB. Circulating asprosin concentrations are increased in type 2 diabetes mellitus and independently associated with fasting glucose and triglyceride. Clin Chim Acta. 2019;489:183–8.
Alan M, Gurlek B, Yilmaz A, Aksit M, Aslanipour B, Gulhan I, et al. Asprosin: a novel peptide hormone related to insulin resistance in women with polycystic ovary syndrome. Gynecol Endocrinol. 2019;35:220–3.
Wang M, Yin CY, Wang L, Liu YS, Li HG, Li M, et al. Serum asprosin concentrations are increased and associated with insulin resistance in children with obesity. Ann Nutr Metab. 2019;75:205–12.
Wang CY, Lin TA, Liu KH, Liao CH, Liu YY, Wu VC, et al. Serum asprosin levels and bariatric surgery outcomes in obese adults. Int J Obes (Lond). 2019;43:1019–25.
Sunnetci Silistre E, Hatipogl HU. Increased serum circulating asprosin levels in children with obesity. Pediatr Int. 2020;62:467–76.
Duerrschmid C, He YL, Wang CM, Li C, Bournat JC, Romere C, et al. Asprosin is a centrally acting orexigenic hormone. Nat Med. 2017;23:1444–53.
Wu J, Bostrom P, Sparks LM, Ye L, Choi JH, Giang AH, et al. Beige adipocytes are a distinct type of thermogenic fat cell in mouse and human. Cell. 2012;150:366–76.
Park A, Kim WK, Bae KH. Distinction of white, beige and brown adipocytes derived from mesenchymal stem cells. World J Stem Cells. 2014;6:33–42.
Kajimura S. Adipose tissue in 2016: Advances in the understanding of adipose tissue biology. Nat Rev Endocrinol. 2017;13:69–70.
Wiecek M, Szymura J, Sproull J, Szygula Z. Decreased blood asprosin in hyperglycemic menopausal women as a result of whole-body cryotherapy regardless of metabolic syndrome. J Clin Med. 2019;8:1428.
Miao YL, Qin HJ, Zhong Y, Huang K, Rao C. Novel adipokine asprosin modulates browning and adipogenesis in white adipose tissue. J Endocrinol. 2021;249:83–93.
Rui LY. Brown and beige adipose tissues in health and disease. Compr Physiol. 2017;7:1281–306.
Acknowledgements
We acknowledge the support from the National Natural Science Foundation of China (81960153 and 82260726), the Natural Science Foundation of Jiangxi Province (20232ACB206061 and 20192BCD40003), the Graduate Innovation Project of Nanchang University (YC2020-B026) and the Undergraduate Innovation Plan Project of China (202110403096).
Author information
Authors and Affiliations
Contributions
Sheng Chen was responsible for conceptualization, formal analysis, investigation, data curation, writing-original draft, writing-review & editing. Wanwan Yuan was responsible for investigation and validation. Qianqian Huang and Xiaowei Xiong was responsible for conceptualization. Chaowen Wang, Wenjing Zeng and Li Wang was responsible for methodology. Yijun Huang, Yeyi Liu and Yan Wang were responsible for validation. Qiren Huang was responsible for conceptualization, writing-review & editing, supervision and funding acquisition. All authors have read and agreed to the published version for the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chen, S., Yuan, W., Huang, Q. et al. Asprosin contributes to pathogenesis of obesity by adipocyte mitophagy induction to inhibit white adipose browning in mice. Int J Obes (2024). https://doi.org/10.1038/s41366-024-01495-6
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41366-024-01495-6