Abstract
Background/objectives
Adipose tissue macrophages (ATM) are key actors in the pathophysiology of obesity-related diseases. They have a unique intermediate M2-M1 phenotype which has been linked to endoplasmic reticulum (ER) stress. We previously reported that human M2 macrophages treated with the ER stress inducer thapsigargin switched to a pro-inflammatory phenotype that depended on the stress protein GRP94. In these conditions, GRP94 promoted cathepsin L secretion and was co-secreted with complement C3. As cathepsin L and complement C3 have been reported to play a role in the pathophysiology of obesity, in this work we studied the involvement of GRP94 in the pro-inflammatory phenotype of ATM.
Methods
GRP94, cathepsin L and C3 expression were analyzed in CD206 + ATM from mice, WT or obesity-resistant transgenic fat-1, fed a high-fat diet (HFD) or a standard diet. GRP94 colocalization with cathepsin L and C3 and its effects were analyzed in human primary macrophages using thapsigargin as a control to induce ER stress and palmitic acid (PA) as a driver of metabolic activation.
Results
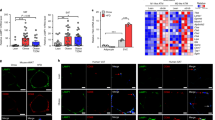
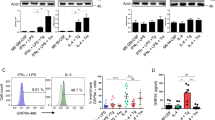
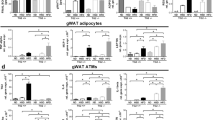
In WT, but not in fat-1 mice, fed a HFD, we observed an increase in crown-like structures consisting of CD206 + pSTAT1+ macrophages showing high expression of GRP94 that colocalized with cathepsin L and C3. In vitro experiments showed that PA favored a M2-M1 switch depending on GRP94. This switch was prevented by omega-3 fatty acids. PA-induced GRP94-cathepsin L colocalization and a decrease in cathepsin L enzymatic activity within the cells (while the enzymatic activity in the extracellular medium was increased). These effects were prevented by the GRP94 inhibitor PU-WS13.
Conclusions
GRP94 is overexpressed in macrophages both in in vivo and in vitro conditions of obesity-associated inflammation and is involved in changing their profile towards a more pro-inflammatory profile. It colocalizes with complement C3 and cathepsin L and modulates cathepsin L activity.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The data generated during and/or analyzed during the current study are available from the corresponding author upon reasonable request.
References
Bluher M. Obesity: global epidemiology and pathogenesis. Nat Rev Endocrinol. 2019;15:288–98.
Collaboration NCDRF. Worldwide trends in body-mass index, underweight, overweight, and obesity from 1975 to 2016: a pooled analysis of 2416 population-based measurement studies in 128.9 million children, adolescents, and adults. Lancet. 2017;390:2627–42.
OMS. WHO. World Health Organization. 2016. ProMED-mail website. Available at: www.who.int/mediacentre/factsheets/fs311/en/.
Zhao X, Gang X, He G, Li Z, Lv Y, Han Q, et al. Obesity increases the severity and mortality of influenza and COVID-19: a systematic review and meta-analysis. Front Endocrinol. 2020;11:595109.
Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006;444:860–7.
Caslin HL, Bhanot M, Bolus WR, Hasty AH. Adipose tissue macrophages: Unique polarization and bioenergetics in obesity. Immunol Rev. 2020;295:101–13.
Russo L, Lumeng CN. Properties and functions of adipose tissue macrophages in obesity. Immunology. 2018;155:407–17.
Lumeng CN, Bodzin JL, Saltiel AR. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Investig. 2007;117:175–84.
Kratz M, Coats BR, Hisert KB, Hagman D, Mutskov V, Peris E, et al. Metabolic dysfunction drives a mechanistically distinct proinflammatory phenotype in adipose tissue macrophages. Cell Metab. 2014;20:614–25.
Cinti S, Mitchell G, Barbatelli G, Murano I, Ceresi E, Faloia E, et al. Adipocyte death defines macrophage localization and function in adipose tissue of obese mice and humans. J Lipid Res. 2005;46:2347–55.
Coats BR, Schoenfelt KQ, Barbosa-Lorenzi VC, Peris E, Cui C, Hoffman A, et al. Metabolically activated adipose tissue macrophages perform detrimental and beneficial functions during diet-induced obesity. Cell Rep. 2017;20:3149–61.
Shan B, Wang X, Wu Y, Xu C, Xia Z, Dai J, et al. The metabolic ER stress sensor IRE1alpha suppresses alternative activation of macrophages and impairs energy expenditure in obesity. Nat Immunol. 2017;18:519–29.
Song L, Kim DS, Gou W, Wang J, Wang P, Wei Z, et al. GRP94 regulates M1 macrophage polarization and insulin resistance. Am J Physiol Endocrinol Metab. 2020;318:E1004–E13.
Chaumonnot K, Masson S, Sikner H, Bouchard A, Baverel V, Bellaye PS, et al. The HSP GRP94 interacts with macrophage intracellular complement C3 and impacts M2 profile during ER stress. Cell Death Dis. 2021;12:114.
Bidu C, Escoula Q, Bellenger S, Spor A, Galan M, Geissler A, et al. The transplantation of omega3 PUFA-altered gut microbiota of fat-1 mice to wild-type littermates prevents obesity and associated metabolic disorders. Diabetes. 2018;67:1512–23.
Kang JX, Wang J, Wu L, Kang ZB. Transgenic mice: fat-1 mice convert n-6 to n-3 fatty acids. Nature. 2004;427:504.
Korbecki J, Bajdak-Rusinek K. The effect of palmitic acid on inflammatory response in macrophages: an overview of molecular mechanisms. Inflamm Res. 2019;68:915–32.
Patel PD, Yan P, Seidler PM, Patel HJ, Sun W, Yang C, et al. Paralog-selective Hsp90 inhibitors define tumor-specific regulation of HER2. Nat Chem Biol. 2013;9:677–84.
Khan S, Luck H, Winer S, Winer DA. Emerging concepts in intestinal immune control of obesity-related metabolic disease. Nat Commun. 2021;12:2598.
Escoula Q, Bellenger S, Narce M, Bellenger J. Docosahexaenoic and eicosapentaenoic acids prevent altered-muc2 secretion induced by palmitic acid by alleviating endoplasmic reticulum stress in LS174T goblet cells. Nutrients. 2019;11:2179.
Yang F, Liu Y, Ren H, Zhou G, Yuan X, Shi X. ER-stress regulates macrophage polarization through pancreatic EIF-2alpha kinase. Cell Immunol. 2019;336:40–7.
Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW Jr. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Investig. 2003;112:1796–808.
Xu H, Barnes GT, Yang Q, Tan G, Yang D, Chou CJ, et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Investig. 2003;112:1821–30.
Lindhorst A, Raulien N, Wieghofer P, Eilers J, Rossi FMV, Bechmann I, et al. Adipocyte death triggers a pro-inflammatory response and induces metabolic activation of resident macrophages. Cell Death Dis. 2021;12:579.
Haka AS, Barbosa-Lorenzi VC, Lee HJ, Falcone DJ, Hudis CA, Dannenberg AJ, et al. Exocytosis of macrophage lysosomes leads to digestion of apoptotic adipocytes and foam cell formation. J Lipid Res. 2016;57:980–92.
Wang Y, Sedlacek AL, Pawaria S, Xu H, Scott MJ, Binder RJ. Cutting edge: the heat shock protein gp96 activates inflammasome-signaling platforms in APCs. J Immunol. 2018;201:2209–14.
Shi H, Kokoeva MV, Inouye K, Tzameli I, Yin H, Flier JS. TLR4 links innate immunity and fatty acid-induced insulin resistance. J Clin Investig. 2006;116:3015–25.
Barbu A, Hamad OA, Lind L, Ekdahl KN, Nilsson B. The role of complement factor C3 in lipid metabolism. Mol Immunol. 2015;67:101–7.
Yang M, Zhang Y, Pan J, Sun J, Liu J, Libby P, et al. Cathepsin L activity controls adipogenesis and glucose tolerance. Nat Cell Biol. 2007;9:970–7.
Choy LN, Rosen BS, Spiegelman BM. Adipsin and an endogenous pathway of complement from adipose cells. J Biol Chem. 1992;267:12736–41.
Liszewski MK, Kolev M, Le Friec G, Leung M, Bertram PG, Fara AF, et al. Intracellular complement activation sustains T cell homeostasis and mediates effector differentiation. Immunity. 2013;39:1143–57.
Weiss-Sadan T, Maimoun D, Oelschlagel D, Kaschani F, Misiak D, Gaikwad H, et al. Cathepsins drive anti-inflammatory activity by regulating autophagy and mitochondrial dynamics in macrophage foam cells. Cell Physiol Biochem. 2019;53:550–72.
Xia W, Lu Z, Chen W, Zhou J, Zhao Y. Excess fatty acids induce pancreatic acinar cell pyroptosis through macrophage M1 polarization. BMC Gastroenterol. 2022;22:72.
Kramer L, Turk D, Turk B. The future of cysteine cathepsins in disease management. Trends Pharmacol Sci. 2017;38:873–98.
Turk V, Stoka V, Vasiljeva O, Renko M, Sun T, Turk B, et al. Cysteine cathepsins: from structure, function and regulation to new frontiers. Biochim Biophys Acta. 2012;1824:68–88.
Vidak E, Javorsek U, Vizovisek M, Turk B. Cysteine cathepsins and their extracellular roles: shaping the microenvironment. Cells. 2019;8:264.
Crewe C, An YA, Scherer PE. The ominous triad of adipose tissue dysfunction: inflammation, fibrosis, and impaired angiogenesis. J Clin Investig. 2017;127:74–82.
Kim M, Lee C, Park J. Extracellular matrix remodeling facilitates obesity-associated cancer progression. Trends Cell Biol. 2022;32:825–34.
Acknowledgements
We thank Nicolas Pernet from the uB Cytometry platform for helping in FACS analysis. This work was supported by grants from the Agence National de la Recherche, Institut Nationale contre le Cancer, Ligue Nationale Contre le Cancer (“Labelled team” to CG) and the Conseil Régional de Bourgogne. The work was also supported by a French Government grant managed by the French National Research Agency under the program ISITE-BFC and LabEX LipSTIC, with reference ANR-11-LABX-0021. We thank the European Union program FEDER for their financial support. FW has a fellowship from ISITE-BFC (Investissements d’Avenir” program, contract ANR-15-IDEX-0003) and from Ningbo University (CHINA).
Author information
Authors and Affiliations
Contributions
Conceptualization: EK. Methodology: FW, VB, KC, AB, SB, JB. Data Analysis: FW, VB, EK. Original Draft Preparation: FW, VB Review & Editing: EK, CG, JB, WZ and MN. Supervision: EK. Funding acquisition: CG and MN. EK is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, F., Baverel, V., Chaumonnot, K. et al. The endoplasmic reticulum stress protein GRP94 modulates cathepsin L activity in M2 macrophages in conditions of obesity-associated inflammation and contributes to their pro-inflammatory profile. Int J Obes (2024). https://doi.org/10.1038/s41366-024-01478-7
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41366-024-01478-7