Abstract
Background
Obesity is a multifactorial condition. Genetic variants, such as the fat mass and obesity related gene (FTO) polymorphism, may increase the vulnerability of developing obesity by disrupting dopamine signaling within the reward network. Yet, the association of obesity, genetic risk of obesity, and structural connectivity of the reward network in adolescents and young adults remains unexplored. We investigate, in adolescents and young adults, the structural connectivity differences in the reward network and at the whole-brain level according to body mass index (BMI) and the FTO rs9939609 polymorphism.
Methods
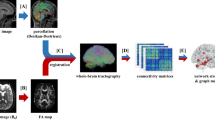
One hundred thirty-two adolescents and young adults (age range: [10, 21] years, BMI z-score range: [−1.76, 2.69]) were included. Genetic risk of obesity was determined by the presence of the FTO A allele. Whole-brain and reward network structural connectivity were analyzed using graph metrics. Hierarchical linear regression was applied to test the association between BMI-z, genetic risk of obesity, and structural connectivity.
Results
Higher BMI-z was associated with higher (B = 0.76, 95% CI = [0.30, 1.21], P = 0.0015) and lower (B = −0.003, 95% CI = [−0.006, −0.00005], P = 0.048) connectivity strength for fractional anisotropy at the whole-brain level and of the reward network, respectively. The FTO polymorphism was not associated with structural connectivity nor with BMI-z.
Conclusions
We provide evidence that, in healthy adolescents and young adults, higher BMI-z is associated with higher connectivity at the whole-brain level and lower connectivity of the reward network. We did not find the FTO polymorphism to correlate with structural connectivity. Future longitudinal studies with larger sample sizes are needed to assess how genetic determinants of obesity change brain structural connectivity and behavior.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Safaei M, Sundararajan EA, Driss M, Boulila W, Shapi’i A. A systematic literature review on obesity: Understanding the causes & consequences of obesity and reviewing various machine learning approaches used to predict obesity. Comput Biol Med. 2021;136:1–17.
Frayling TM, Timpson NJ, Weedon MN, Zeggini E, Freathy RM, Lindgren CM, et al. A Common Variant in the FTO Gene Is Associated with Body Mass Index and Predisposes to Childhood and Adult Obesity. Science. 2007;316:889–94.
Loos RJF, Yeo GSH. The genetics of obesity: from discovery to biology. Nat Rev Genet. 2022;23:120–33.
Speakman JR. The “Fat Mass and Obesity Related” (FTO) gene: Mechanisms of Impact on Obesity and Energy Balance. Curr Obes Rep. 2015;4:73–91.
Hess ME, Hess S, Meyer KD, Verhagen LAW, Koch L, Brönneke HS, et al. The fat mass and obesity associated gene (Fto) regulates activity of the dopaminergic midbrain circuitry. Nat Neurosci. 2013;16:1042–8.
Marqués-Iturria I, Scholtens LH, Garolera M, Pueyo R, García-García I, González-Tartiere P, et al. Affected connectivity organization of the reward system structure in obesity. Neuroimage. 2015;111:100–6.
Edwin Thanarajah S, Hanssen R, Melzer C, Tittgemeyer M. Increased meso-striatal connectivity mediates trait impulsivity in FTO variant carriers. Front Endocrinol. 2023;14:1130203.
Adise S, Allgaier N, Laurent J, Hahn S, Chaarani B, Owens M, et al. Multimodal brain predictors of current weight and weight gain in children enrolled in the ABCD study ®. Dev Cogn Neurosci. 2021;49:1–14.
Beyer F, Zhang R, Scholz M, Wirkner K, Loeffler M, Stumvoll M, et al. Higher BMI, but not obesity-related genetic polymorphisms, correlates with lower structural connectivity of the reward network in a population-based study. Int J Obes. 2021;45:491–501.
Gupta A, Mayer EA, Sanmiguel CP, Van Horn JD, Woodworth D, Ellingson BM, et al. Patterns of brain structural connectivity differentiate normal weight from overweight subjects. NeuroImage Clin. 2015;7:506–17.
Riederer JW, Shott ME, Deguzman M, Pryor TL, Frank GKW. Understanding neuronal architecture in obesity through analysis of white matter connection strength. Front Hum Neurosci. 2016;10:271.
García-García I, Morys F, Dagher A. Nucleus accumbens volume is related to obesity measures in an age-dependent fashion. J Neuroendocrinol. 2020;32:1–12.
Lancaster TM, Ihssen I, Brindley LM, Linden DE. Preliminary evidence for genetic overlap between body mass index and striatal reward response. Transl Psychiatry. 2018;8:1–9.
Olivo G, Latini F, Wiemerslage L, Larsson EM, Schiöth HB. Disruption of accumbens and thalamic white matter connectivity revealed by diffusion tensor tractography in young men with genetic risk for obesity. Front Hum Neurosci. 2018;12:1–10.
Lugo-Candelas C, Pang Y, Lee S, Cha J, Hong S, Ranzenhofer L, et al. Differences in brain structure and function in children with the FTO obesity-risk allele. Obes Sci Pract. 2020;6:409–24.
Sawyer SM, Azzopardi PS, Wickremarathne D, Patton GC. The age of adolescence. Lancet Child Adolesc Heal. 2018;2:223–8.
Prats-Soteras X, Jurado MA, Ottino-González J, García-García I, Segura B, Caldú X, et al. Inflammatory agents partially explain associations between cortical thickness, surface area, and body mass in adolescents and young adulthood. Int J Obes. 2020;44:1487–96.
Prunell-Castañé A, Jurado MÁ, Ottino-González J, Prats-Soteras X, Sánchez Garre C, Cano Marco N, et al. Beyond BMI: cardiometabolic measures as predictors of impulsivity and white matter changes in adolescents. Brain Struct Funct. 2023;228:751–60.
Must A, Anderson SE. Body mass index in children and adolescents: Considerations for population-based applications. Int J Obes. 2006;30:590–4.
Cole TJ, Lobstein T. Extended international (IOTF) body mass index cut-offs for thinness, overweight and obesity. Pediatr Obes. 2012;7:284–94.
Organization WH. World Health Organization. Obesity and overweight. Available from: https://www.who.int/en/news-room/fact-sheets/detail/obesity-and-overweight
Veraart J, Novikov DS, Christiaens D, Ades-aron B, Sijbers J, Fieremans E. Denoising of diffusion MRI using random matrix theory. Neuroimage. 2016;142:394–406.
Bhushan C, Haldar JP, Joshi AA, Leahy RM. Correcting susceptibility-induced distortion in diffusion-weighted mri using constrained nonrigid registration. Signal Inf Process Assoc Annu Summit Conf APSIPA Asia Pac. 2012.
Andersson JLR, Skare S, Ashburner J. How to correct susceptibility distortions in spin-echo echo-planar images: application to diffusion tensor imaging. Neuroimage. 2003;20:870–88.
Andersson JLR, Sotiropoulos SN. An integrated approach to correction for off-resonance effects and subject movement in diffusion MR imaging. Neuroimage. 2016;125:1063–78.
Andersson JLR, Graham MS, Zsoldos E, Sotiropoulos SN. Incorporating outlier detection and replacement into a non-parametric framework for movement and distortion correction of diffusion MR images. Neuroimage. 2016;141:556–72.
Bastiani M, Cottaar M, Fitzgibbon SP, Suri S, Alfaro-Almagro F, Sotiropoulos SN, et al. Automated quality control for within and between studies diffusion MRI data using a non-parametric framework for movement and distortion correction. Neuroimage. 2019;184:801–12.
Yeh FC, Wedeen VJ, Tseng WYI. Generalized q-sampling imaging. IEEE Trans Med Imaging. 2010;29:1626–35.
Yeh FC, Verstynen TD, Wang Y, Fernández-Miranda JC, Tseng WYI. Deterministic diffusion fiber tracking improved by quantitative anisotropy. PLoS One. 2013;8:1–16.
Fortin JP, Parker D, Tunç B, Watanabe T, Elliott MA, Ruparel K, et al. Harmonization of multi-site diffusion tensor imaging data. Neuroimage. 2017;161:149–70.
da Silva TER, Andrade NL, de Oliveira Cunha D, Leão-Cordeiro JAB, Vilanova-Costa CAST, Silva AMTC. The FTO rs9939609 polymorphism and obesity risk in teens: Evidence-based meta-analysis. Obes Res Clin Pract. 2018;12:432–7.
Khera AV, Chaffin M, Wade KH, Zahid S, Brancale J, Xia R, et al. Polygenic prediction of weight and obesity trajectories from birth to adulthood. Cell. 2019;177:587–96.
Ganeff IMM, Bos MM, Van Heemst D, Noordam R. BMI-associated gene variants in FTO and cardiometabolic and brain disease: obesity or pleiotropy? Physiol Genomics. 2019;51:311–22.
Rutters F, Nieuwenhuizen AG, Bouwman F, Mariman E, Westerterp-plantenga MS. Associations between a single nucleotide polymorphism of the FTO Gene (rs9939609) and obesity-related characteristics over time during puberty in a Dutch children cohort. J Clin Endocrinol Metab. 2011;96:939–42.
Foraita R, Günther F, Gwozdz W, Reisch LA, Russo P, Lauria F, et al. Does the FTO gene interact with the socioeconomic status on the obesity development among young European children? Results from the IDEFICS study. Int J Obes. 2015;39:1–6.
Rask-Andersen M, Karlsson T, Ek WE, Johansson Å. Gene-environment interaction study for BMI reveals interactions between genetic factors and physical activity, alcohol consumption and socioeconomic status. PLoS Genet. 2017;13:1–20.
Mahmoud AM. An overview of epigenetics in obesity: the role of lifestyle and therapeutic interventions. Int J Mol Sci. 2022;23:1341.
Tan Z, Hu Y, Ji G, Li G, Ding Y, Zhang W, et al. Alterations in functional and structural connectivity of basal ganglia network in patients with obesity. Brain Topogr. 2022;35:453–63.
Augustijn MJCM, Di Biase MA, Zalesky A, Van Acker L, De Guchtenaere A, D’Hondt E, et al. Structural connectivity and weight loss in children with obesity: a study of the “connectobese.”. Int J Obes. 2019;43:2309–21.
Meng X, Huang D, Ao H, Wang X, Gao X. Food cue recruits increased reward processing and decreased inhibitory control processing in the obese/overweight: an activation likelihood estimation meta-analysis of fMRI studies. Obes Res Clin Pract. 2020;14:127–35.
Carbine KA, Duraccio KM, Hedges-Muncy A, Barnett KA, Kirwan CB, Jensen CD. White matter integrity disparities between normal-weight and overweight/obese adolescents: an automated fiber quantification tractography study. Brain Imaging Behav. 2020;14:308–19.
Ma J, Mcglade EC, Id RSH, Lyoo IK, Renshaw F, Yurgelun-todd DA. Overweight/Obesity-related microstructural alterations of the fimbria-fornix in the ABCD study: the role of aerobic physical activity. PLoS One. 2023;18:1–16.
Melhorn SJ, Askren MK, Chung WK, Kratz M, Bosch TA, Tyagi V, et al. FTO genotype impacts food intake and corticolimbic activation. Am J Clin Nutr. 2018;107:145–54.
De Groot C, Felius A, Trompet S, De Craen AJM, Blauw GJ, Van Buchem MA, et al. Association of the fat mass and obesity-associated gene risk allele, rs9939609A, and reward-related brain structures. Obesity. 2015;23:2118–22.
Galvan A. Adolescent development of the reward system. Front Hum Neurosci. 2010;4:1–9.
Reichelt AC. Adolescent maturational transitions in the prefrontal cortex and dopamine signaling as a risk factor for the development of obesity and high fat/high sugar diet induced cognitive deficits. Front Behav Neurosci. 2016;10:1–17.
Geier CF. Adolescent cognitive control and reward processing: implications for risk taking and substance use. Horm Behav. 2013;64:333–42.
Blakemore SJ. Imaging brain development: the adolescent brain. Neuroimage. 2012;61:397–406.
Sadler JR, Thapaliya G, Ranganath K, Gabay A, Chen L, Smith KR, et al. Paediatric obesity and metabolic syndrome associations with cognition and the brain in youth: Current evidence and future directions. Pediatr Obes. 2023;18:1–27.
Stiles J, Jernigan TL. The basics of brain development. Neuropsychol Rev. 2010;20:327–48.
Loos RJF, Yeo GSH. The bigger picture of FTO - the first GWAS-identified obesity gene. Nat Rev Endocrinol. 2014;10:51–61.
Jiang Y, Mei H, Lin Q, Wang J, Liu S, Wang G, et al. Interaction effects of FTO rs9939609 polymorphism and lifestyle factors on obesity indices in early adolescence. Obes Res Clin Pract. 2019;13:352–7.
Acknowledgements
The authors thank Isabel García-García and Jonatan Ottino-González their support. The authors also thank all the participants that made this project possible. We acknowledge the Centres de Recerca de Catalunya (CERCA) Program/Generalitat de Catalunya, the Institute of Neurosciences, the Institute of Biomedical Research August Pi i Sunyer (IDIBAPS), the Consorci Sanitari de Terrassa, and the Hospital de Terrassa. We acknowledge the following funding sources, which were not involved in the study conceptualization, interpretation or writing: APC received a Ph.D. scholarship from the Spanish Ministry of Science, Innovation and Universities (PRE2019-087430). MAJ and MG received Grants from the Spanish Ministry of Economy, Industry and Competitiveness (PSI2017- 86536-C2-1-R and PSI2017-86536-C2-2-R, respectively), funded by MCIN/AEI/https://doi.org/10.13039/501100011033 and the European Regional Development Fund (ERDF). MAJ, MG, XC and APC have additionally received funding from the Departament d’Innovació, Universitats i Empresa, Generalitat de Catalunya (2021SGR0801).
Author information
Authors and Affiliations
Contributions
APC, MAJ, MG, FB, and VW contributed to study design and conception. APC, IH, CSG, XC participated in data acquisition. APC performed the analyses. APC, MAJ, and FB contributed to results interpretation. APC wrote the original manuscript. All authors critically reviewed the manuscript, approved its final version for publishing, and agreed to be accountable for all aspects of such work.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Prunell-Castañé, A., Beyer, F., Witte, V. et al. From the reward network to whole-brain metrics: structural connectivity in adolescents and young adults according to body mass index and genetic risk of obesity. Int J Obes 48, 567–574 (2024). https://doi.org/10.1038/s41366-023-01451-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41366-023-01451-w