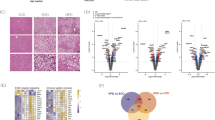
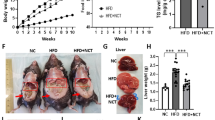
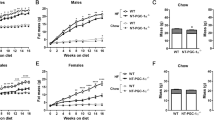
Abstract
Objectives
Obesity and non-alcoholic fatty liver disease (NAFLD) are major health concerns. The circadian rhythm is an autonomous and intrinsic timekeeping system closely associated with energy metabolism and obesity. Thus, this study explored the role of brain and muscle aryl hydrocarbon receptor nuclear translocator-like1 (BMAL1), a circadian clock regulator, in the development of obesity and NAFLD.
Methods
We generated BMAL1 knockout (BMAL1 KO) mice to imitate circadian rhythm disruption. The study comprised three groups from the same litter: BMAL1 KO mice fed a high-fat diet (to establish obesity and NAFLD phenotypes), wild-type mice fed normal chow, and wild-type mice fed a high-fat diet. The metabolic and NAFLD phenotypes were assessed via physiological measurements and histological examinations. Quantitative polymerase chain reaction and western blotting were used to identify and validate changes in the signaling pathways responsible for the altered NAFLD phenotypes in the wild-type and BMAL1 KO mice.
Results
BMAL1 depletion protected against obesity and metabolic disorders induced by a high-fat diet. BMAL1 depletion also prevented hepatic steatosis and inhibited cluster of differentiation 36 and peroxisome proliferator-activated receptor gamma (i.e., PPARγ) expression.
Conclusions
BMAL1 plays an important role in the development of obesity and NAFLD and, thus, is a potential therapeutic target for these conditions.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
Data will be made available on request.
References
Blüher M. Obesity: global epidemiology and pathogenesis. Nat Rev Endocrinol. 2019;15:288–98.
Abarca-Gómez L, Abdeen ZA, Hamid ZA, Abu-Rmeileh NM, Acosta-Cazares B, Acuin C, et al. Worldwide trends in body-mass index, underweight, overweight, and obesity from 1975 to 2016: a pooled analysis of 2416 population-based measurement studies in 128.9 million children, adolescents, and adults. Lancet. 2017;390:2627–42.
Riazi K, Azhari H, Charette JH, Underwood FE, King JA, Afshar EE, et al. The prevalence and incidence of NAFLD worldwide: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2022;7:851–61.
Paik JM, Golabi P, Younossi Y, Mishra A, Younossi ZM. Changes in the global burden of chronic liver diseases from 2012 to 2017: the growing impact of NAFLD. Hepatology. 2020;72:1605–16.
Pafili K, Roden M. Nonalcoholic fatty liver disease (NAFLD) from pathogenesis to treatment concepts in humans. Mol Metab. 2021;50:101122.
Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease-meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64:73–84.
Friedman SL, Neuschwander-Tetri BA, Rinella M, Sanyal AJ. Mechanisms of NAFLD development and therapeutic strategies. Nat Med. 2018;24:908–22.
Sanyal AJ. Past, present and future perspectives in nonalcoholic fatty liver disease. Nat Rev Gastroenterol Hepatol. 2019;16:377–86.
Gnocchi D, Custodero C, Sabbà C, Mazzocca A. Circadian rhythms: a possible new player in non-alcoholic fatty liver disease pathophysiology. J Mol Med. 2019;97:741–59.
Pilorz V, Helfrich-Förster C, Oster H. The role of the circadian clock system in physiology. Pflugers Arch. 2018;470:227–39.
Bass J, Lazar MA. Circadian time signatures of fitness and disease. Science. 2016;354:994–9.
Saran AR, Dave S, Zarrinpar A. Circadian rhythms in the pathogenesis and treatment of fatty liver disease. Gastroenterology. 2020;158:1948–.e1.
Shetty A, Hsu JW, Manka PP, Syn WK. Role of the circadian clock in the metabolic syndrome and nonalcoholic fatty liver disease. Dig Dis Sci. 2018;63:3187–206.
Patke A, Young MW, Axelrod S. Molecular mechanisms and physiological importance of circadian rhythms. Nat Rev Mol Cell Biol. 2020;21:67–84.
Marcheva B, Ramsey KM, Buhr ED, Kobayashi Y, Su H, Ko CH, et al. Disruption of the clock components CLOCK and BMAL1 leads to hypoinsulinaemia and diabetes. Nature. 2010;466:627–31.
Birky TL, Bray MS. Understanding circadian gene function: animal models of tissue-specific circadian disruption. IUBMB Life. 2014;66:34–41.
Shimba S, Ogawa T, Hitosugi S, Ichihashi Y, Nakadaira Y, Kobayashi M, et al. Deficient of a clock gene, brain and muscle Arnt-like protein-1 (BMAL1), induces dyslipidemia and ectopic fat formation. PLoS ONE. 2011;6:e25231.
Jouffe C, Weger BD, Martin E, Atger F, Weger M, Gobet C, et al. Disruption of the circadian clock component BMAL1 elicits an endocrine adaption impacting on insulin sensitivity and liver disease. Proc Natl Acad Sci USA. 2022;119:e2200083119.
Shen Q, Yang Y, Liu W, Wang M, Shao Y, Xu B, et al. Organ-specific alterations in circadian genes by vertical sleeve gastrectomy in an obese diabetic mouse model. Sci Bull. 2017;62:467–9.
Lim YC, Hoe VCW, Darus A, Bhoo-Pathy N. Association between night-shift work, sleep quality and metabolic syndrome. Occup Environ Med. 2018;75:716–23.
Gan Y, Yang C, Tong X, Sun H, Cong Y, Yin X, et al. Shift work and diabetes mellitus: a meta-analysis of observational studies. Occup Environ Med. 2015;72:72–8.
Kondratov RV, Kondratova AA, Gorbacheva VY, Vykhovanets OV, Antoch MP. Early aging and age-related pathologies in mice deficient in BMAL1, the core componentof the circadian clock. Genes Dev. 2006;20:1868–73.
Shi SQ, Ansari TS, Mcguinness OP, Wasserman DH, Johnson CH. Circadian disruption leads to insulin resistance and obesity. Curr Biol. 2013;23:372–81.
Yang G, Zhang J, Jiang T, Monslow J, Tang SY, Todd L, et al. BMAL1 deletion in myeloid cells attenuates atherosclerotic lesion development and restrains abdominal aortic aneurysm formation in hyperlipidemic mice. Arterioscler Thromb Vasc Biol. 2020;40:1523–32.
Kitchen GB, Cunningham PS, Poolman TM, Iqbal M, Maidstone R, Baxter M, et al. The clock gene BMAL1 inhibits macrophage motility, phagocytosis, and impairs defense against pneumonia. Proc Natl Acad Sci USA. 2020;117:1543–51.
Rui L. Energy metabolism in the liver. Compr Physiol. 2014;4:177–97.
Rada P, González-Rodríguez Á, García-Monzón C, Valverde ÁM. Understanding lipotoxicity in NAFLD pathogenesis: is CD36 a key driver? Cell Death Dis. 2020;11:802.
Zhou J, Febbraio M, Wada T, Zhai Y, Kuruba R, He J, et al. Hepatic fatty acid transporter CD36 is a common target of LXR, PXR, and PPARgamma in promoting steatosis. Gastroenterology. 2008;134:556–67.
Xiao Y, Kim M, Lazar MA. Nuclear receptors and transcriptional regulation in non-alcoholic fatty liver disease. Mol Metab. 2021;50:101119.
Ko CW, Qu J, Black DD, Tso P. Regulation of intestinal lipid metabolism: current concepts and relevance to disease. Nat Rev Gastroenterol Hepatol. 2020;17:169–83.
Jones JG. Hepatic glucose and lipid metabolism. Diabetologia. 2016;59:1098–103.
Grabner GF, Xie H, Schweiger M, Zechner R. Lipolysis: cellular mechanisms for lipid mobilization from fat stores. Nat Metab. 2021;3:1445–65.
Acknowledgements
This study was supported by the National Natural Science Foundation of China (grant numbers 81800751, 81970458, and 82100882).
Author information
Authors and Affiliations
Contributions
WL and QS conceived, designed, and supervised the study. CZ, HC, BX, YS, QY and RH conducted the study and analyzed the results. CZ and ZZ wrote and revised the manuscript. QS provided financial support.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhan, C., Chen, H., Zhang, Z. et al. BMAL1 deletion protects against obesity and non-alcoholic fatty liver disease induced by a high-fat diet. Int J Obes 48, 469–476 (2024). https://doi.org/10.1038/s41366-023-01435-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41366-023-01435-w