Abstract
Background
Overweight and obesity are the consequence of a sustained positive energy balance. Twin studies show high heritability rates pointing to genetics as one of the principal risk factors. By 2022, genomic studies led to the identification of almost 300 obesity-associated variants that could help to fill the gap of the high heritability rates. The endocannabinoid system is a critical regulator of metabolism for its effects on the central nervous system and peripheral tissues. Fatty acid amide hydrolase (FAAH) is a key enzyme in the inactivation of one of the two endocannabinoids, anandamide, and of its congeners. The rs324420 variant within the FAAH gene is a nucleotide missense change at position 385 from cytosine to adenine, resulting in a non-synonymous amino acid substitution from proline to threonine in the FAAH enzyme. This change increases sensitivity to proteolytic degradation, leading to reduced FAAH levels and increased levels of anandamide, associated with obesity-related traits. However, association studies of this variant with metabolic parameters have found conflicting results. This work aims to perform a systematic review of the existing literature on the association of the rs324420 variant in the FAAH gene with obesity and its related traits.
Methods
A literature search was conducted in PubMed, Web of Science, and Scopus. A total of 645 eligible studies were identified for the review.
Results/Conclusions
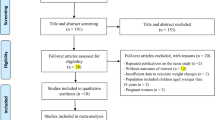
After the identification, duplicate elimination, title and abstract screening, and full-text evaluation, 28 studies were included, involving 28 183 individuals. We show some evidence of associations between the presence of the variant allele and higher body mass index, waist circumference, fat mass, and waist-to-hip ratio levels and alterations in glucose and lipid homeostasis. However, this evidence should be taken with caution, as many included studies did not report a significant difference between genotypes. These discordant results could be explained mainly by the pleiotropy of the endocannabinoid system, the increase of other anandamide-like mediators metabolized by FAAH, and the influence of gene-environment interactions. More research is necessary to study the endocannabinoidomic profiles and their association with metabolic diseases.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
Data availability
The extracted data that support the findings of this study is available from the corresponding author upon request.
References
Smith KB, Smith MS. Obesity statistics. Prim Care Clin Off Pract. 2016;43:121–35. https://doi.org/10.1016/j.pop.2015.10.001.
Bray GA, Kim KK, Wilding JPH. On behalf of the World Obesity Federation. Obesity: a chronic relapsing progressive disease process. A position statement of the World Obesity Federation: Position Paper. Obes Rev. 2017;18:715–23. https://doi.org/10.1111/obr.12551.
Dobbs R, Sawers C, Thompson F, Manyika J, Woetzel J, Child P, et al. Overcoming Obesity: An Initial Economic Analysis. Jakarta, Indonesia: McKinsey Global Institute; 2014.
Prospective Studies Collaboration. Body-mass index and cause-specific mortality in 900 000 adults: collaborative analyses of 57 prospective studies. The Lancet. 2009;373:1083–96. https://doi.org/10.1016/S0140-6736(09)60318-4.
Stunkard AJ, Harris JR, Pedersen NL, McClearn GE. The body-mass index of twins who have been reared apart. N Engl J Med. 1990;322:1483–7. https://doi.org/10.1056/NEJM199005243222102.
Elks CE, den Hoed M, Zhao JH, Sharp SJ, Wareham NJ, Loos RJF, et al. Variability in the Heritability of Body Mass Index: A Systematic Review and Meta-Regression. Front Endocrinol. 2012;3. https://doi.org/10.3389/fendo.2012.00029.
Maes HHM, Neale MC, Eaves LJ. Genetic and environmental factors in relative body weight and human adiposity. Behav Genet. 1997;27:325–51. https://doi.org/10.1023/A:1025635913927.
Wu Y, Duan H, Tian X, Xu C, Wang W, Jiang W, et al. Genetics of obesity traits: a bivariate genome-wide association analysis. Front Genet. 2018;9:179 https://doi.org/10.3389/fgene.2018.00179.
EMBL-EBI. GWAS Catalog. Trait Obes 2021. https://www.ebi.ac.uk/gwas/efotraits/EFO_0001073 (accessed October 1, 2021).
Yang Q, Khoury MJ, Friedman J, Little J, Flanders WD. How many genes underlie the occurrence of common complex diseases in the population? Int J Epidemiol. 2005;34:1129–37. https://doi.org/10.1093/ije/dyi130.
Speliotes EK, Willer CJ, Berndt SI, Monda KL, Thorleifsson G, Jackson AU, et al. Association analyses of 249,796 individuals reveal 18 new loci associated with body mass index. Nat Genet. 2010;42:937–48. https://doi.org/10.1038/ng.686.
Grimm ER, Steinle NI. Genetics of eating behavior: established and emerging concepts: Nutrition Reviews©. Nutr Rev. 2011;69:52–60. https://doi.org/10.1111/j.1753-4887.2010.00361.x.
Qi L, Cho YA. Gene-environment interaction and obesity. Nutr Rev. 2008;66:684–94. https://doi.org/10.1111/j.1753-4887.2008.00128.x.
Howlett AC. International Union of Pharmacology. XXVII. Classification of Cannabinoid Receptors. Pharmacol Rev. 2002;54:161–202. https://doi.org/10.1124/pr.54.2.161.
Di Marzo V. Endocannabinoids: synthesis and degradation. Rev. Physiol. Biochem. Pharmacol., 160, Berlin, Heidelberg: Springer Berlin Heidelberg; 2006, 1–24. https://doi.org/10.1007/112_0505.
Silvestri C, Di Marzo V. The endocannabinoid system in energy homeostasis and the etiopathology of metabolic disorders. Cell Metab. 2013;17:475–90. https://doi.org/10.1016/j.cmet.2013.03.001.
Simon V, Cota D. MECHANISMS IN ENDOCRINOLOGY: Endocannabinoids and metabolism: past, present and future. Eur J Endocrinol. 2017;176:R309–24. https://doi.org/10.1530/EJE-16-1044.
Pagotto U, Marsicano G, Cota D, Lutz B, Pasquali R. The emerging role of the endocannabinoid system in endocrine regulation and energy balance. Endocr Rev. 2006;27:73–100. https://doi.org/10.1210/er.2005-0009.
Dainese E, Oddi S, Simonetti M, Sabatucci A, Angelucci CB, Ballone A, et al. The endocannabinoid hydrolase FAAH is an allosteric enzyme. Sci Rep. 2020;10:2292 https://doi.org/10.1038/s41598-020-59120-1.
Rakotoarivelo V, Sihag J, Flamand N. Role of the endocannabinoid system in the adipose tissue with focus on energy metabolism. Cells. 2021;10:1279 https://doi.org/10.3390/cells10061279.
Kathuria S, Gaetani S, Fegley D, Valiño F, Duranti A, Tontini A, et al. Modulation of anxiety through blockade of anandamide hydrolysis. Nat Med. 2003;9:76–81. https://doi.org/10.1038/nm803.
Wan M, Cravatt BF, Ring HZ, Zhang X, Francke U. Conserved chromosomal location and genomic structure of human and mouse fatty-acid amide hydrolase genes and evaluation ofclasper as a candidate neurological mutation. Genomics. 1998;54:408–14. https://doi.org/10.1006/geno.1998.5597.
Cravatt BF, Giang DK, Mayfield SP, Boger DL, Lerner RA, Gilula NB. Molecular characterization of an enzyme that degrades neuromodulatory fatty-acid amides. Nature. 1996;384:83–7. https://doi.org/10.1038/384083a0.
Sipe JC, Chiang K, Gerber AL, Beutler E, Cravatt BF. A missense mutation in human fatty acid amide hydrolase associated with problem drug use. Proc Natl Acad Sci. 2002;99:8394–9. https://doi.org/10.1073/pnas.082235799.
Chiang KP. Reduced cellular expression and activity of the P129T mutant of human fatty acid amide hydrolase: evidence for a link between defects in the endocannabinoid system and problem drug use. Hum Mol Genet. 2004;13:2113–9. https://doi.org/10.1093/hmg/ddh216.
Sipe JC, Waalen J, Gerber A, Beutler E. Overweight and obesity associated with a missense polymorphism in fatty acid amide hydrolase (FAAH). Int J Obes. 2005;29:755–9. https://doi.org/10.1038/sj.ijo.0802954.
Tang L, Ye H, Hong Q, Chen F, Wang Q, Xu L, et al. Meta-analyses between 18 candidate genetic markers and overweight/obesity. Diagn Pathol. 2014;9:56 https://doi.org/10.1186/1746-1596-9-56.
Doris JM, Millar SA, Idris I, O’Sullivan SE. Genetic polymorphisms of the endocannabinoid system in obesity and diabetes. Diabetes Obes Metab. 2019;21:382–7. https://doi.org/10.1111/dom.13504.
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021:n71. https://doi.org/10.1136/bmj.n71.
Graffelman J. Exploring Diallelic Genetic Markers: The HardyWeinberg Package. J Stat Softw 2015;64. https://doi.org/10.18637/jss.v064.i03.
Jensen DP, Andreasen CH, Andersen MK, Hansen L, Eiberg H, Borch-Johnsen K, et al. The functional Pro129Thr variant of the FAAH gene is not associated with various fat accumulation phenotypes in a population-based cohort of 5801 whites. J Mol Med. 2007;85:445–9. https://doi.org/10.1007/s00109-006-0139-0.
Aberle J, Fedderwitz I, Klages N, George E, Beil F. Genetic variation in two proteins of the endocannabinoid system and their influence on body mass index and metabolism under low fat diet. Horm Metab Res. 2007;39:395–7. https://doi.org/10.1055/s-2007-977694.
Sarzani R, Bordicchia M, Salvi F, Cola G, Franchi E, Battistoni I, et al. A Human Fatty Acid Amide Hydrolase (FAAH) Functional Gene Variant Is Associated With Lower Blood Pressure in Young Males. Am J Hypertens. 2008;21:960–3. https://doi.org/10.1038/ajh.2008.198.
Monteleone P, Tortorella A, Martiadis V, Di Filippo C, Canestrelli B, Maj M. The cDNA 385C to A missense polymorphism of the endocannabinoid degrading enzyme fatty acid amide hydrolase (FAAH) is associated with overweight/obesity but not with binge eating disorder in overweight/obese women. Psychoneuroendocrinology. 2008;33:546–50. https://doi.org/10.1016/j.psyneuen.2008.01.004.
Papazoglou D, Panagopoulos I, Papanas N, Gioka T, Papadopoulos T, Papathanasiou P, et al. The Fatty Acid Amide Hydrolase (FAAH) Pro129Thr Polymorphism is not Associated with Severe Obesity in Greek Subjects. Horm Metab Res. 2008;40:907–10. https://doi.org/10.1055/s-0028-1087169.
Durand E, Lecoeur C, Delplanque J, Benzinou M, Degraeve F, Boutin P, et al. Evaluating the Association of FAAH Common Gene Variation with Childhood, Adult Severe Obesity and Type 2 Diabetes in the French Population. Obes Facts. 2008;1:305–9. https://doi.org/10.1159/000178157.
de Luis DA, Gonzalez Sagrado M, Aller R, Izaola O, Conde R, Romero E. C358A missense polymorphism of the endocannabinoid-degrading enzyme fatty acid amide hydrolase (FAAH) and visfatin levels in obese females. Int J Obes. 2010;34:511–5. https://doi.org/10.1038/ijo.2009.283.
de Luis DA. Relation of C358A polymorphism of the endocannabinoid degrading enzyme fatty acid amide hydrolase (faah) with obesity and insulin resistance. Nutr Hosp 2010:1–2. https://doi.org/10.3305/nh.2010.25.6.4843.
de Luis DA, Sagrado MG, Aller R, Izaola O, Conde R, Romero E. C358A missense polymorphism of the endocannabinoid degrading enzyme fatty acid amide hydrolase (FAAH) and insulin resistance in patients with diabetes mellitus type 2. Diabetes Res Clin Pract. 2010;88:76–80. https://doi.org/10.1016/j.diabres.2009.12.019.
de Luis DA, Sagrado MG, Pacheco D, Terroba MC, Martin T, Cuellar L, et al. Effects of C358A missense polymorphism of the endocannabinoid degrading enzyme fatty acid amide hydrolase on weight loss and cardiovascular risk factors 1 year after biliopancreatic diversion surgery. Surg Obes Relat Dis. 2010;6:516–20. https://doi.org/10.1016/j.soard.2010.01.005.
De Luis DA, González Sagrado M, Aller R, Izaola O, Conde R, Romero E. Polimorfismo C358A de la enzima «amida hidrolasa de ácidos grasos» y los niveles de adipocitoquinas en obesos mórbidos. Endocrinol Nutr. 2010;57:54–9. https://doi.org/10.1016/j.endonu.2010.01.001.
Deluis DA, Sagrado MG, Aller R, Izaola O, Conde R. Effects of C358A missense polymorphism of the degrading enzyme fatty acid amide hydrolase on weight loss, adipocytokines, and insulin resistance after 2 hypocaloric diets. Metabolism. 2010;59:1387–92. https://doi.org/10.1016/j.metabol.2009.12.029.
Monteleone P, Milano W, Petrella C, Canestrelli B, Maj M. Endocannabinoid Pro129Thr FAAH Functional Polymorphism But Not 1359G/A CNR1 Polymorphism Is Associated With Antipsychotic-Induced Weight Gain. J Clin Psychopharmacol. 2010;30:441–5. https://doi.org/10.1097/JCP.0b013e3181e742c5.
Müller TD, Brönner G, Wandolski M, Carrie J, Nguyen TT, Greene BH, et al. Mutation screen and association studies for the fatty acid amide hydrolase (FAAH) gene and early onset and adult obesity. BMC Med Genet. 2010;11:2 https://doi.org/10.1186/1471-2350-11-2.
de Luis DA, Gonzalez Sagrado M, Aller R, Izaola O, Conde R. Effects of C358A missense polymorphism of the endocannabinoid degrading enzyme fatty acid amide hydrolase on weight loss after a hypocaloric diet. Metabolism. 2011;60:730–4. https://doi.org/10.1016/j.metabol.2010.07.007.
de Luis DA, Aller R, Izaola O, Conde R, Sagrado MG, Primo D, et al. Relationship among metabolic syndrome, C358A polymorphism of the endocannabinoid degrading enzyme fatty acid amide hydrolase (FAAH) and insulin resistance. J Diabetes Complications. 2012;26:328–32. https://doi.org/10.1016/j.jdiacomp.2012.04.002.
Knoll N, Volckmar A-L, Pütter C, Scherag A, Kleber M, Hebebrand J, et al. The Fatty Acid Amide Hydrolase (FAAH) Gene Variant rs324420 AA/AC is not Associated with Weight Loss in a 1-Year Lifestyle Intervention for Obese Children and Adolescents. Horm Metab Res. 2012;44:75–7. https://doi.org/10.1055/s-0031-1291306.
de Luis DA, Izaola O, Aller R, de La Fuente B, Pacheco D. Effects of C358A polymorphism of the endocannabinoid degrading enzyme fatty acid amide hydrolase (FAAH) on weight loss, adipocytokines levels, and insulin resistance after a high polyunsaturated fat diet in obese patients. J Endocrinol Invest. 2013;36:965–9. https://doi.org/10.1007/BF03346760.
De Luis DA, Aller R, Izaola O, Conde R, de la Fuente B, Sagrado MG. Genetic variation in the endocannabinoid degrading enzyme fatty acid amide hydrolase (FAAH) and their influence on weight loss and insulin resistance under a high monounsaturated fat hypocaloric diet. J Diabetes Complications. 2013;27:235–9. https://doi.org/10.1016/j.jdiacomp.2012.11.001.
Grolmusz V, Stenczer B, Fekete T, Szendei G, Patócs A, Rácz K, et al. Lack of Association between C385A Functional Polymorphism of the Fatty Acid Amide Hydrolase Gene and Polycystic Ovary Syndrome. Exp Clin Endocrinol Diabetes. 2013;121:338–42. https://doi.org/10.1055/s-0033-1337941.
Zhang Y, Sonnenberg GE, Baye TM, Littrell J, Gunnell J, DeLaForest A, et al. Obesity-related dyslipidemia associated with FAAH, independent of insulin response, in multigenerational families of Northern European descent. Pharmacogenomics. 2009;10:1929–39. https://doi.org/10.2217/pgs.09.122.
Lieb W, Manning AK, Florez JC, Dupuis J, Cupples LA, McAteer JB, et al. Variants in the CNR1 and the FAAH Genes and Adiposity Traits in the Community. Obesity. 2009;17:755–60. https://doi.org/10.1038/oby.2008.608.
Thethi TK, Sigel A, Japa S, Katalenich B, Liu S, Nguyen T, et al. Racial and sex differences in the polymorphisms of the endocannabinoid receptor genes in obesity. J Diabetes Complications. 2020;34:107682 https://doi.org/10.1016/j.jdiacomp.2020.107682.
Sierra-Ruelas E, Torres-Castillo N, Vizmanos B, Campos-Pérez W, Lopez-Cortes OD, Di Marzo V, et al. FAAH Pro129Thr Variant Is Associated with Increased Cholesterol Levels in Normal-Weight Metabolically Unhealthy Subjects. Metabolic Syndrome and Related Disorders 2023:met.2023.0014. https://doi.org/10.1089/met.2023.0014.
Zeng J, Li J, Huang G. 385 C/A polymorphism of the fatty acid amide hydrolase gene is associated with metabolic syndrome in the Chinese Han population. Arch Med Sci. 2011;3:423–7. https://doi.org/10.5114/aoms.2011.23406.
Yagin NL, Aliasgari F, Aliasgharzadeh S, Mahdavi R, Akbarzadeh M. The influence of the fatty acid amide hydrolase 385C>A single nucleotide polymorphisms on obesity susceptibility. Mol Biol Rep. 2019;46:5049–55. https://doi.org/10.1007/s11033-019-04956-8.
Martins CJ, Genelhu V, Pimentel MM, Celoria BM, Mangia RF, Aveta T, et al. Circulating Endocannabinoids and the Polymorphism 385C>A in Fatty Acid Amide Hydrolase (FAAH) Gene May Identify the Obesity Phenotype Related to Cardiometabolic Risk: A Study Conducted in a Brazilian Population of Complex Interethnic Admixture. Plos One. 2015;10:e0142728 https://doi.org/10.1371/journal.pone.0142728.
Touriño C, Oveisi F, Lockney J, Piomelli D, Maldonado R. FAAH deficiency promotes energy storage and enhances the motivation for food. Int J Obes. 2010;34:557–68. https://doi.org/10.1038/ijo.2009.262.
Jamshidi N, Taylor DA. Anandamide administration into the ventromedial hypothalamus stimulates appetite in rats: Special Report. Br J Pharmacol. 2001;134:1151–4. https://doi.org/10.1038/sj.bjp.0704379.
Karaliota S, Siafaka-Kapadai A, Gontinou C, Psarra K, Mavri-Vavayanni M. Anandamide increases the differentiation of rat adipocytes and causes PPARgamma and CB1 receptor upregulation. Obes Silver Spring Md. 2009;17:1830–8. https://doi.org/10.1038/oby.2009.177.
Williams CM, Kirkham TC. Anandamide induces overeating: mediation by central cannabinoid (CB1) receptors. Psychopharmacology (Berl). 1999;143:315–7. https://doi.org/10.1007/s002130050953.
Matheson J, Zhou XMM, Bourgault Z, Le Foll B. Potential of Fatty Acid Amide Hydrolase (FAAH), Monoacylglycerol Lipase (MAGL), and Diacylglycerol Lipase (DAGL) Enzymes as Targets for Obesity Treatment: A Narrative Review. Pharm Basel Switz 2021;14. https://doi.org/10.3390/ph14121316.
Sipe JC, Scott TM, Murray S, Harismendy O, Simon GM, Cravatt BF, et al. Biomarkers of endocannabinoid system activation in severe obesity. PloS One. 2010;5:e8792 https://doi.org/10.1371/journal.pone.0008792.
Hillard CJ. Circulating Endocannabinoids: From Whence Do They Come and Where are They Going? Neuropsychopharmacology. 2018;43:155–72. https://doi.org/10.1038/npp.2017.130.
Di Marzo V. New approaches and challenges to targeting the endocannabinoid system. Nat Rev Drug Discov. 2018;17:623–39. https://doi.org/10.1038/nrd.2018.115.
Ramírez-Orozco RE, García-Ruiz R, Morales P, Villalón CM, Villafán-Bernal JR, Marichal-Cancino BA. Potential metabolic and behavioural roles of the putative endocannabinoid receptors GPR18, GPR55 and GPR119 in feeding. Curr Neuropharmacol. 2019;17:947–60. https://doi.org/10.2174/1570159X17666190118143014.
Lichtman AH, Shelton CC, Advani T, Cravatt BF. Mice lacking fatty acid amide hydrolase exhibit a cannabinoid receptor-mediated phenotypic hypoalgesia. Pain. 2004;109:319–27. https://doi.org/10.1016/j.pain.2004.01.022.
Chang L, Luo L, Palmer JA, Sutton S, Wilson SJ, Barbier AJ, et al. Inhibition of fatty acid amide hydrolase produces analgesia by multiple mechanisms: Mechanisms of analgesia by FAAH inhibition. Br J Pharmacol. 2006;148:102–13. https://doi.org/10.1038/sj.bjp.0706699.
Huggins JP, Smart TS, Langman S, Taylor L, Young T. An efficient randomised, placebo-controlled clinical trial with the irreversible fatty acid amide hydrolase-1 inhibitor PF-04457845, which modulates endocannabinoids but fails to induce effective analgesia in patients with pain due to osteoarthritis of the knee. Pain. 2012;153:1837–46. https://doi.org/10.1016/j.pain.2012.04.020.
Bermudez-Silva F, Suarez J, Baixeras E, Cobo N, Bautista D, Cuesta-Munoz A, et al. Presence of functional cannabinoid receptors in human endocrine pancreas. Diabetologia. 2008;51:476–87. https://doi.org/10.1007/s00125-007-0890-y.
Di Marzo V. The endocannabinoid system in obesity and type 2 diabetes. Diabetologia. 2008;51:1356–67. https://doi.org/10.1007/s00125-008-1048-2.
Liu YL, Connoley IP, Wilson CA, Stock MJ. Effects of the cannabinoid CB1 receptor antagonist SR141716 on oxygen consumption and soleus muscle glucose uptake in Lepob/Lepob mice. Int J Obes. 2005;29:183–7. https://doi.org/10.1038/sj.ijo.0802847.
Di Marzo V, Verrijken A, Hakkarainen A, Petrosino S, Mertens I, Lundbom N, et al. Role of insulin as a negative regulator of plasma endocannabinoid levels in obese and nonobese subjects. Eur J Endocrinol. 2009;161:715–22. https://doi.org/10.1530/EJE-09-0643.
Vaitheesvaran B, Yang L, Hartil K, Glaser S, Yazulla S, Bruce JE, et al. Peripheral Effects of FAAH Deficiency on Fuel and Energy Homeostasis: Role of Dysregulated Lysine Acetylation. PLoS ONE. 2012;7:e33717 https://doi.org/10.1371/journal.pone.0033717.
Liu J, Cinar R, Xiong K, Godlewski G, Jourdan T, Lin Y, et al. Monounsaturated fatty acids generated via stearoyl CoA desaturase-1 are endogenous inhibitors of fatty acid amide hydrolase. Proc Natl Acad Sci USA. 2013;110:18832–7. https://doi.org/10.1073/pnas.1309469110.
Pagano C, Rossato M, Vettor R. Endocannabinoids, Adipose Tissue and Lipid Metabolism. J Neuroendocrinol. 2008;20:124–9. https://doi.org/10.1111/j.1365-2826.2008.01690.x.
Krott LM, Piscitelli F, Heine M, Borrino S, Scheja L, Silvestri C, et al. Endocannabinoid regulation in white and brown adipose tissue following thermogenic activation. J Lipid Res. 2016;57:464–73. https://doi.org/10.1194/jlr.M065227.
Brown WH, Gillum MP, Lee H-Y, Camporez JPG, Zhang X, Jeong JK, et al. Fatty acid amide hydrolase ablation promotes ectopic lipid storage and insulin resistance due to centrally mediated hypothyroidism. Proc Natl Acad Sci USA. 2012;109:14966–71. https://doi.org/10.1073/pnas.1212887109.
Bazwinsky-Wutschke I, Zipprich A, Dehghani F. Endocannabinoid System in Hepatic Glucose Metabolism, Fatty Liver Disease, and Cirrhosis. Int J Mol Sci. 2019;20:2516 https://doi.org/10.3390/ijms20102516.
Pu S, Eck P, Jenkins DJA, Connelly PW, Lamarche B, Kris-Etherton PM, et al. Interactions between dietary oil treatments and genetic variants modulate fatty acid ethanolamides in plasma and body weight composition. Br J Nutr. 2016;115:1012–23. https://doi.org/10.1017/S0007114515005425.
Després J-P, Ross R, Boka G, Alméras N, Lemieux I. Effect of Rimonabant on the High-Triglyceride/ Low–HDL-Cholesterol Dyslipidemia, Intraabdominal Adiposity, and Liver Fat: The ADAGIO-Lipids Trial. Arterioscler Thromb Vasc Biol. 2009;29:416–23. https://doi.org/10.1161/ATVBAHA.108.176362.
Poirier B, Bidouard J-P, Cadrouvele C, Marniquet X, Staels B, O’Connor SE, et al. The anti-obesity effect of rimonabant is associated with an improved serum lipid profile. Diabetes Obes Metab. 2005;7:65–72. https://doi.org/10.1111/j.1463-1326.2004.00374.x.
Di Marzo V, Goparaju SK, Wang L, Liu J, Bátkai S, Járai Z, et al. Leptin-regulated endocannabinoids are involved in maintaining food intake. Nature. 2001;410:822–5. https://doi.org/10.1038/35071088.
Balsevich G, Sticht M, Bowles NP, Singh A, Lee TTY, Li Z, et al. Role for fatty acid amide hydrolase (FAAH) in the leptin-mediated effects on feeding and energy balance. Proc Natl Acad Sci USA. 2018;115:7605–10. https://doi.org/10.1073/pnas.1802251115.
Park HT, Cho SH, Cho GJ, Shin JH, Hong SC, Kim T, et al. Relationship between serum adipocytokine levels and metabolic syndrome in menopausal women. Gynecol Endocrinol. 2009;25:27–31. https://doi.org/10.1080/09513590802404021.
Muccioli GG, Naslain D, Bäckhed F, Reigstad CS, Lambert DM, Delzenne NM, et al. The endocannabinoid system links gut microbiota to adipogenesis. Mol Syst Biol. 2010;6:392 https://doi.org/10.1038/msb.2010.46.
Little TJ, Cvijanovic N, DiPatrizio NV, Argueta DA, Rayner CK, Feinle-Bisset C, et al. Plasma endocannabinoid levels in lean, overweight, and obese humans: relationships to intestinal permeability markers, inflammation, and incretin secretion. Am J Physiol-Endocrinol Metab. 2018;315:E489–95. https://doi.org/10.1152/ajpendo.00355.2017.
Khan RN, Maner-Smith K, A. Owens J, Barbian ME, Jones RM, R. Naudin C. At the heart of microbial conversations: endocannabinoids and the microbiome in cardiometabolic risk. Gut Microbes. 2021;13:1911572 https://doi.org/10.1080/19490976.2021.1911572.
Maccarrone M, De Petrocellis L, Bari M, Fezza F, Salvati S, Di Marzo V, et al. Lipopolysaccharide Downregulates Fatty Acid Amide Hydrolase Expression and Increases Anandamide Levels in Human Peripheral Lymphocytes. Arch Biochem Biophys. 2001;393:321–8. https://doi.org/10.1006/abbi.2001.2500.
Lake KD, Compton DR, Varga K, Martin BR, Kunos G. Cannabinoid-induced hypotension and bradycardia in rats mediated by CB1-like cannabinoid receptors. J Pharmacol Exp Ther. 1997;281:1030–7.
Sarzani R. Endocannabinoids, Blood Pressure and the Human Heart. J Neuroendocrinol. 2008;20:58–62. https://doi.org/10.1111/j.1365-2826.2008.01677.x.
Bátkai S, Pacher P, Osei-Hyiaman D, Radaeva S, Liu J, Harvey-White J, et al. Endocannabinoids Acting at Cannabinoid-1 Receptors Regulate Cardiovascular Function in Hypertension. Circulation. 2004;110:1996–2002. https://doi.org/10.1161/01.CIR.0000143230.23252.D2.
Soria-Gómez E, Matias I, Rueda-Orozco PE, Cisneros M, Petrosino S, Navarro L, et al. Pharmacological enhancement of the endocannabinoid system in the nucleus accumbens shell stimulates food intake and increases c-Fos expression in the hypothalamus: Endocannabinoids and food intake. Br J Pharmacol. 2007;151:1109–16. https://doi.org/10.1038/sj.bjp.0707313.
Soria-Gómez E, Bellocchio L, Reguero L, Lepousez G, Martin C, Bendahmane M, et al. The endocannabinoid system controls food intake via olfactory processes. Nat Neurosci. 2014;17:407–15. https://doi.org/10.1038/nn.3647.
Di Marzo V, Maccarrone M. FAAH and anandamide: is 2-AG really the odd one out? Trends Pharmacol Sci. 2008;29:229–33. https://doi.org/10.1016/j.tips.2008.03.001.
Fezza F, Bari M, Florio R, Talamonti E, Feole M, Maccarrone M. Endocannabinoids, related compounds and their metabolic routes. Molecules. 2014;19:17078–106. https://doi.org/10.3390/molecules191117078.
Simard M, Archambault A-S, Lavoie J-PC, Dumais É, Di Marzo V, Flamand N. Biosynthesis and metabolism of endocannabinoids and their congeners from the monoacylglycerol and N-acyl-ethanolamine families. Biochemical Pharmacology. 2022;205:115261 https://doi.org/10.1016/j.bcp.2022.115261.
Starowicz K, Makuch W, Korostynski M, Malek N, Slezak M, Zychowska M, et al. Full inhibition of spinal FAAH leads to TRPV1-mediated analgesic effects in neuropathic rats and possible lipoxygenase-mediated remodeling of anandamide metabolism. PLoS ONE. 2013;8:e60040 https://doi.org/10.1371/journal.pone.0060040.
Gatta L, Piscitelli F, Giordano C, Boccella S, Lichtman A, Maione S, et al. Discovery of prostamide F2α and its role in inflammatory pain and dorsal horn nociceptive neuron hyperexcitability. PLoS ONE. 2012;7:e31111 https://doi.org/10.1371/journal.pone.0031111.
Author information
Authors and Affiliations
Contributions
ODL-C, FT-S, ES-R, and BV were responsible for designing the study protocol. ODL-C, FT-S, conducted literature searches, screening, full-text review, data extraction and risk of bias analysis. E-S-R helped resolve discrepancies in the systematic review process. ODL-C performed the analyses, interpretated the data, constructed the tables and figures, and drafted the article. All authors critically revised the article for important intellectual content and approved the final version to be published.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lopez-Cortes, O.D., Trujillo-Sánchez, F., Sierra-Ruelas, E. et al. Association between the FAAH C385A variant (rs324420) and obesity-related traits: a systematic review. Int J Obes 48, 188–201 (2024). https://doi.org/10.1038/s41366-023-01428-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41366-023-01428-9