Abstract
Background and Objectives
Overweight and obesity is a complex condition resulting from unbalanced energy homeostasis among various organs. However, systemic abnormalities in overweight and obese people are seldom explored in vivo by metabolic imaging techniques. The aim of this study was to determine metabolic abnormities throughout the body in overweight and obese adults using total-body positron emission tomography (PET) glucose uptake imaging.
Methods
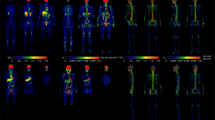
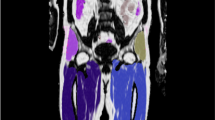
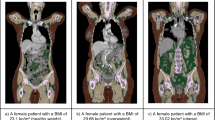
Thirty normal weight subjects [body mass index (BMI) < 25 kg/m2, 55.47 ± 13.94 years, 16 men and 14 women], and 26 overweight and obese subjects [BMI ≥ 25 kg/m2, 52.38 ± 9.52 years, 21 men and 5 women] received whole-body 18F-fluorodeoxyglucose PET imaging using the uEXPLORER. Whole-body standardized uptake value normalized by lean body mass (SUL) images were calculated. Metabolic networks were constructed based on the whole-body SUL images using covariance network approach. Both group-level and individual-level network differences between normal weight and overweight/obese subjects were evaluated. Correlation analysis was conducted between network properties and BMI for the overweight/obese subjects.
Results
Compared with normal weight subjects, overweight/obese subjects exhibited altered network connectivity strength in four network nodes, namely the pancreas (p = 0.033), spleen (p = 0.021), visceral adipose tissue (VAT) (p = 1.12 × 10−5) and bone (p = 0.021). Network deviations of overweight/obese subjects from the normal weight were positively correlated with BMI (r = 0.718, p = 3.64 × 10−5). In addition, overweight/obese subjects experienced altered connections between organs, and some of the altered connections, including pancreas-right lung and VAT-bilateral lung connections were significantly correlated with BMI.
Conclusion
Overweight/obese individuals exhibit metabolic alterations in organ level, and altered metabolic interactions at the systemic level. The proposed approach using total-body PET imaging can reveal individual metabolic variability and metabolic deviations between organs, which would open up a new path for understanding metabolic alterations in overweight and obesity.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Engin A. The definition and prevalence of obesity and metabolic syndrome. Adv Exp Med Biol. 2017;960:1–17. https://doi.org/10.1007/978-3-319-48382-5_1
Dai H, Alsalhe TA, Chalghaf N, Riccò M, Bragazzi NL, Wu J. The global burden of disease attributable to high body mass index in 195 countries and territories, 1990-2017: an analysis of the Global Burden of Disease Study. PLoS Med. 2020;17:e1003198 https://doi.org/10.1371/journal.pmed.1003198
Nejat EJ, Polotsky AJ, Pal L. Predictors of chronic disease at midlife and beyond–the health risks of obesity. Maturitas. 2010;65:106–11. https://doi.org/10.1016/j.maturitas.2009.09.006
U Din M, Raiko J, Saari T, Saunavaara V, Kudomi N, Solin O, et al. Human brown fat radiodensity indicates underlying tissue composition and systemic metabolic health. J Clin Endocrinol Metab. 2017;102:2258–67. https://doi.org/10.1210/jc.2016-2698
Iozzo P, Guiducci L, Guzzardi MA, Pagotto U. Brain PET imaging in obesity and food addiction: current evidence and hypothesis. Obes Facts. 2012;5:155–64. https://doi.org/10.1159/000338328
Goossens GH. The metabolic phenotype in obesity: fat mass, body fat distribution, and adipose tissue function. Obes Facts. 2017;10:207–15. https://doi.org/10.1159/000471488
Oliveira AL, Azevedo DC, Bredella MA, Stanley TL, Torriani M. Visceral and subcutaneous adipose tissue FDG uptake by PET/CT in metabolically healthy obese subjects. Obesity. 2015;23:286–9. https://doi.org/10.1002/oby.20957
Virtanen KA, Lönnroth P, Parkkola R, Peltoniemi P, Asola M, Viljanen T, et al. Glucose uptake and perfusion in subcutaneous and visceral adipose tissue during insulin stimulation in nonobese and obese humans. J Clin Endocrinol Metab. 2002;87:3902–10. https://doi.org/10.1210/jcem.87.8.8761
Green AL, Bagci U, Hussein S, Kelly PV, Muzaffar R, Neuschwander-Tetri BA, et al. Brown adipose tissue detected by PET/CT imaging is associated with less central obesity. Nucl Med Commun. 2017;38:629–35. https://doi.org/10.1097/MNM.0000000000000691
Finlayson G. Food addiction and obesity: unnecessary medicalization of hedonic overeating. Nat Rev Endocrinol. 2017;13:493–8. https://doi.org/10.1038/nrendo.2017.61
Volkow ND, Wang GJ, Telang F, Fowler JS, Goldstein RZ, Alia-Klein N, et al. Inverse association between BMI and prefrontal metabolic activity in healthy adults. Obesity. 2009;17:60–65. https://doi.org/10.1038/oby.2008.469
Val-Laillet D, Aarts E, Weber B, Ferrari M, Quaresima V, Stoeckel LE, et al. Neuroimaging and neuromodulation approaches to study eating behavior and prevent and treat eating disorders and obesity. Neuroimage Clin. 2015;8:1–31. https://doi.org/10.1016/j.nicl.2015.03.016
Bakkum MJ, Danad I, Romijn MA, Stuijfzand WJ, Leonora RM, Tulevski II, et al. The impact of obesity on the relationship between epicardial adipose tissue, left ventricular mass and coronary microvascular function. Eur J Nucl Med Mol Imaging. 2015;42:1562–73. https://doi.org/10.1007/s00259-015-3087-5
Liu C, Shao M, Lu L, Zhao C, Qiu L, Liu Z. Obesity, insulin resistance and their interaction on liver enzymes. PLoS One. 2021;16:e0249299 https://doi.org/10.1371/journal.pone.0249299
Honka H, Hannukainen JC, Tarkia M, Karlsson H, Saunavaara V, Salminen P, et al. Pancreatic metabolism, blood flow, and β-cell function in obese humans. J Clin Endocrinol Metab. 2014;99:E981–90. https://doi.org/10.1210/jc.2013-4369
Merz KE, Thurmond DC. Role of skeletal muscle in insulin resistance and glucose uptake. Compr Physiol. 2020;10:785–809. https://doi.org/10.1002/cphy.c190029
Vandenberghe S, Moskal P, Karp JS. State of the art in total body PET. EJNMMI Phys. 2020;7:35. https://doi.org/10.1186/s40658-020-00290-2
Lu W, Cheng Z, Xie X, Li K, Duan Y, Li M, et al. An atlas of glucose uptake across the entire human body as measured by the total-body PET/CT scanner: a pilot study, Life Metab. 2023. (in press) https://doi.org/10.1093/lifemeta/loac030
Sun T, Wang Z, Wu Y, Gu F, Li X, Bai Y, et al. Identifying the individual metabolic abnormities from a systemic perspective using whole-body PET imaging. Eur J Nucl Med Mol Imaging. 2022;49:2994–3004. https://doi.org/10.1007/s00259-022-05832-7
Hatta T. Handedness and the brain: a review of brain-imaging techniques. Magn Reson Med Sci. 2007;6:99–12.
Caballero B. Humans against obesity: who will win? Adv Nutr. 2019;10:S4–S9.
Sarikaya I, Albatineh AN, Sarikaya A. Revisiting weight-normalized SUV and lean-body-mass-normalized SUV in PET studies. J Nucl Med Technol. 2020;48:163–7. https://doi.org/10.2967/jnmt.119.233353
Hume R. Prediction of lean body mass from height and weight. J Clin Pathol. 1966;19:389–91. https://doi.org/10.1136/jcp.19.4.389
Liu Z, Palaniyappan L, Wu X, Zhang K, Du J, Zhao Q, et al. Resolving heterogeneity in schizophrenia through a novel systems approach to brain structure: individualized structural covariance network analysis. Mol Psychiatry. 2021;26:7719–31. https://doi.org/10.1038/s41380-021-01229-4
Rorsman P, Ashcroft FM. Pancreatic β-Cell electrical activity and insulin secretion: of mice and men. Physiol Rev. 2018;98:117–14. https://doi.org/10.1152/physrev.00008.2017
Di Bonito P, Pacifico L, Chiesa C, Valerio G, Miraglia Del Giudice E, Maffeis C, et al. Impaired fasting glucose and impaired glucose tolerance in children and adolescents with overweight/obesity. J Endocrinol Investig. 2017;40:409–16. https://doi.org/10.1007/s40618-016-0576-8
Kim HG, Han J. Obesity and pancreatic diseases. Korean J Gastroenterol. 2012;59:35–39. https://doi.org/10.4166/kjg.2012.59.1.35
Gotoh K, Inoue M, Masaki T, Chiba S, Shimasaki T, Ando H, et al. A novel anti-inflammatory role for spleen-derived interleukin-10 in obesity-induced inflammation in white adipose tissue and liver. Diabetes. 2012;61:1994–2003. https://doi.org/10.2337/db11-1688
Gheorghe A, Pérez de Heredia F, Hunsche C, Redondo N, Díaz LE, Hernández O, et al. Oxidative stress and immunosenescence in spleen of obese mice can be reversed by 2-hydroxyoleic acid. Exp Physiol. 2017;102:533–44. https://doi.org/10.1113/EP086157
Hou J, He C, He W, Yang M, Luo X, Li C. Obesity and bone health: a complex link. Front Cell Dev Biol. 2020;8:600181. https://doi.org/10.1113/EP086157
Cao JJ. Effects of obesity on bone metabolism. J Orthop Surg Res. 2011;6:30 https://doi.org/10.1186/1749-799X-6-30
Ambati S, Yu P, McKinney EC, Kandasamy MK, Hartzell D, Baile CA, et al. Adipocyte nuclei captured from VAT and SAT. BMC Obes. 2016;3:35. https://doi.org/10.1186/s40608-016-0112-6
Dixon AE, Peters U. The effect of obesity on lung function. Expert Rev Respir Med. 2018;12:755–67. https://doi.org/10.1080/17476348.2018.1506331
Melo LC, Silva MA, Calles AC. Obesity and lung function: a systematic review. Einstein. 2014;12:120–5. https://doi.org/10.1590/s1679-45082014rw2691
Cuspidi C, Rescaldani M, Sala C, Grassi G. Left-ventricular hypertrophy and obesity: a systematic review and meta-analysis of echocardiographic studies. J Hypertens. 2014;32:16–25. https://doi.org/10.1097/HJH.0b013e328364fb58
de Simone G, Izzo R, De Luca N, Gerdts E. Left ventricular geometry in obesity: is it what we expect. Nutr Metab Cardiovasc Dis. 2013;23:905–12. https://doi.org/10.1016/j.numecd.2013.06.012
Funding
This work was supported by the Science and Technology Funding from Jinan (grant number: 2020GXRC018), Academic Promotion Program of Shandong First Medical University (grant number: 2019QL009), Natural Science Foundation of Shandong Province (Grant number: ZR2023QH109), and Taishan Scholars Program of Shandong Province (grant number: TS201712065).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection were performed by YD, KL and ZC data processing and analysis were performed by WL and JQ The first draft of the manuscript was written by WL. All authors commented on previous versions of the manuscript and all authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors have no conflicts of interest to declare that are relevant to the content of this article. All authors commented on previous versions of the manuscript and all authors read and approved the final manuscript.
Ethics approval
This study received approval from the Institutional Review Board of Shandong First Medical University in accordance with the Declaration of Helsinki.
Consent to participate
Prior to PET/CT scan, all subjects gave their written informed consent.
Consent for publication
All authors commented on previous versions of the manuscript and all authors read and approved the final manuscript.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lu, W., Duan, Y., Li, K. et al. Metabolic interactions between organs in overweight and obesity using total-body positron emission tomography. Int J Obes 48, 94–102 (2024). https://doi.org/10.1038/s41366-023-01394-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41366-023-01394-2