Abstract
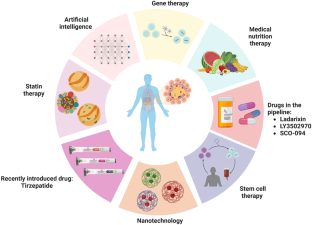
Diabetes is a serious health issue that causes a progressive dysregulation of carbohydrate metabolism due to insufficient insulin hormone, leading to consistently high blood glucose levels. According to the epidemiological data, the prevalence of diabetes has been increasing globally, affecting millions of individuals. It is a long-term condition that increases the risk of various diseases caused by damage to small and large blood vessels. There are two main subtypes of diabetes: type 1 and type 2, with type 2 being the most prevalent. Genetic and molecular studies have identified several genetic variants and metabolic pathways that contribute to the development and progression of diabetes. Current treatments include gene therapy, stem cell therapy, statin therapy, and other drugs. Moreover, recent advancements in therapeutics have also focused on developing novel drugs targeting these pathways, including incretin mimetics, SGLT2 inhibitors, and GLP-1 receptor agonists, which have shown promising results in improving glycemic control and reducing the risk of complications. However, these treatments are often expensive, inaccessible to patients in underdeveloped countries, and can have severe side effects. Peptides, such as glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide-1 (GLP-1), are being explored as a potential therapy for diabetes. These peptides are postprandial glucose-dependent pancreatic beta-cell insulin secretagogues and have received much attention as a possible treatment option. Despite these advances, diabetes remains a major health challenge, and further research is needed to develop effective treatments and prevent its complications. This review covers various aspects of diabetes, including epidemiology, genetic and molecular basis, and recent advancements in therapeutics including herbal and synthetic peptides.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
Data availability
The datasets utilized and/or analyzed during this study are included in the manuscript.
References
American Diabetes A. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2009;32:S62–7.
Fareed M, Chauhan W, Fatma R, Din I, Afzal M, Ahmed Z. Next-generation sequencing technologies in diabetes research. Diabetes Epidemiol Manag. 2022;7:100097.
Lampasona V, Petrone A, Tiberti C, Capizzi M, Spoletini M, di Pietro S, et al. Zinc transporter 8 antibodies complement GAD and IA-2 antibodies in the identification and characterization of adult-onset autoimmune diabetes: Non Insulin Requiring Autoimmune Diabetes (NIRAD) 4. Diabetes Care. 2010;33:104–8.
Galicia-Garcia U, Benito-Vicente A, Jebari S, Larrea-Sebal A, Siddiqi H. et al. Pathophysiology of type 2 diabetes mellitus. Int J Mol Sci. 2020;21:6275.
Plows JF, Stanley JL, Baker PN, Reynolds CM, Vickers MH. The pathophysiology of gestational diabetes mellitus. Int J Mol Sci. 2018;19:3342.
Skoczek D, Dulak J, Kachamakova-Trojanowska N. Maturity onset diabetes of the young-new approaches for disease modelling. Int J Mol Sci. 2021;14:7553.
Cole JB, Florez JC. Genetics of diabetes mellitus and diabetes complications. Nat Rev Nephrol. 2020;16:377–90.
Ikle JM, Gloyn AL. 100 YEARS OF INSULIN: a brief history of diabetes genetics: insights for pancreatic beta-cell development and function. J Endocrinol. 2021;250:R23–R35.
Redondo M, Yu L, Hawa M, Mackenzie T, Pyke D, Eisenbarth G, et al. Heterogeneity of type I diabetes: analysis of monozygotic twins in Great Britain and the United States. Diabetologia. 2001;44:354–62.
Mychaleckyj JC, Noble JA, Moonsamy PV, Carlson JA, Varney MD, Post J, et al. HLA genotyping in the international Type 1 Diabetes Genetics Consortium. Clinical Trials. 2010;7:S75–S87.
Ounissi-Benkalha H, Polychronakos C. The molecular genetics of type 1 diabetes: new genes and emerging mechanisms. Trends Mol Med. 2008;14:268–75.
Noble JA, Valdes AM. Genetics of the HLA region in the prediction of type 1 diabetes. Curr Diab Rep. 2011;11:533–42.
Sanchez-Mazas A, Meyer D. The relevance of HLA sequencing in population genetics studies. J Immunol Res. 2014;2014.
Buhler S, Sanchez-Mazas A. HLA DNA sequence variation among human populations: molecular signatures of demographic and selective events. PloS One. 2011;6:e14643.
Eisenbarth GSJD. Banting Lecture 2009: An unfinished journey: molecular pathogenesis to prevention of type 1A diabetes. Diabetes. 2010;59:759.
Wyatt RC, Lanzoni G, Russell MA, Gerling I, Richardson SJ. What the HLA-I!-Classical and non-classical HLA Class I and their potential roles in type 1 diabetes. Curr Diab Rep. 2019;19:159.
Redondo MJ, Steck AK. Pugliese AJPd. Genetics of type 1. Diabetes. 2018;19:346–53.
Noble JA, Erlich HA. Genetics of type 1 diabetes. Cold Spring Harb Perspect Med. 2012;2:a007732.
Laine AP, Valta M, Toppari J, et al. Non-HLA Gene Polymorphisms in the Pathogenesis of Type 1 Diabetes: Phase and Endotype Specific Effects. Front Immunol. 2022;13:909020.
Pociot F, Akolkar B, Concannon P, et al. Genetics of type 1 diabetes: what’s next?. Diabetes. 2010;59:1561–71.
Pérez de Nanclares G, Bilbao JR, Calvo B, Vitoria JC, Vázquez F, Castaño L. 5′‐Insulin gene VNTR polymorphism is specific for type 1 diabetes: no association with celiac or Addison’s disease. Ann N Y Acad Sci. 2003;1005:319–23.
Pugliese A, Zeller M, Fernandez A, Zalcberg LJ, Bartlett RJ, Ricordi C, et al. The insulin gene is transcribed in the human thymus and transcription levels correlate with allelic variation at the INS VNTR-IDDM2 susceptibility locus for type 1 diabetes. Nat Genet. 1997;15:293–7.
Bennett S, Lucassen A, Gough S, Powell E, Undlien D, Pritchard L, et al. Susceptibility to human type 1 diabetes at IDDM2 is determined by tandem repeat variation at the insulin gene minisatellite locus. Nat Genet. 1995;9:284–92.
Wang J, Liu L, Ma J, Sun F, Zhao Z, Gu M. Common variants on cytotoxic T lymphocyte antigen-4 polymorphisms contributes to type 1 diabetes susceptibility: evidence based on 58 studies. PloS One. 2014;9:e85982.
Kavvoura FK, Ioannidis JP. CTLA-4 gene polymorphisms and susceptibility to type 1 diabetes mellitus: a HuGE review and meta-analysis. Am J Epidemiol. 2005;162:3–16.
Bottini N, Musumeci L, Alonso A, Rahmouni S, Nika K, Rostamkhani M, et al. A functional variant of lymphoid tyrosine phosphatase is associated with type I diabetes. Nat Genet. 2004;36:337–8.
Begovich AB, Carlton VE, Honigberg LA, Schrodi SJ, Chokkalingam AP, Alexander HC, et al. A missense single-nucleotide polymorphism in a gene encoding a protein tyrosine phosphatase (PTPN22) is associated with rheumatoid arthritis. Am J Hum Genet. 2004;75:330–7.
Lempainen J, Laine AP, Hammais A, et al. Non-HLA gene effects on the disease process of type 1 diabetes: From HLA susceptibility to overt disease. J Autoimmun. 2015;61:45–53.
Steck AK, Dong F, Wong R, Fouts A, Liu E, Romanos J, et al. Improving prediction of type 1 diabetes by testing non‐HLA genetic variants in addition to HLA markers. Pediatr Diabetes. 2014;15:355–62.
Lempainen J, Hermann R, Veijola R, Simell O, Knip M, Ilonen J. Effect of the PTPN22 and INS risk genotypes on the progression to clinical type 1 diabetes after the initiation of β-cell autoimmunity. Diabetes. 2012;61:963–966.
Bugawan TL, Mirel DB, Valdes AM, Panelo A, Pozzilli P, Erlich HA. Association and interaction of the IL4R, IL4, and IL13 loci with type 1 diabetes among Filipinos. Am J Hum Genet. 2003;72:1505–14.
Noble JA. Immunogenetics of type 1 diabetes: a comprehensive review. J Autoimmun. 2015;64:101–12.
Shastry A, Sedimbi S, Rajalingam R, Nikitina‐Zake L, Rumba I, Wigzell H, et al. Combination of KIR 2DL2 and HLA‐C1 (Asn80) confers susceptibility to type 1 diabetes in Latvians. Int J of Immunogenet. 2008;35:439–46.
Sabater L, Ferrer-Francesch X, Sospedra M, Caro P, Juan M, Pujol-Borrell R. Insulin alleles and autoimmune regulator (AIRE) gene expression both influence insulin expression in the thymus. J Autoimmun. 2005;25:312–8.
Visperas A, Vignali DA. Are regulatory T cells defective in type 1 diabetes and can we fix them? J Immunol. 2016;197:3762–70.
Flanagan SE, Haapaniemi E, Russell MA, Caswell R, Allen HL, De Franco E, et al. Activating germline mutations in STAT3 cause early-onset multi-organ autoimmune disease. Nat Genet. 2014;46:812–4.
Udler MS, Kim J, von Grotthuss M, Bonàs-Guarch S, Cole JB, Chiou J, et al. Type 2 diabetes genetic loci informed by multi-trait associations point to disease mechanisms and subtypes: a soft clustering analysis. PLoS Med. 2018;15:e1002654.
Mahajan A, Taliun D, Thurner M, Robertson NR, Torres JM, Rayner NW, et al. Fine-mapping type 2 diabetes loci to single-variant resolution using high-density imputation and islet-specific epigenome maps. Nat Genet. 2018;50:1505–13.
Udler MS. Type 2 diabetes: multiple genes, multiple diseases. Curr Diab Rep. 2019;19:1–9.
Ge Y, Onengut-Gumuscu S, Quinlan AR, Mackey AJ, Wright JA, Buckner JH, et al. Targeted deep sequencing in multiple-affected sibships of European ancestry identifies rare deleterious variants in PTPN22 that confer risk for type 1 diabetes. Diabetes. 2016;65:794–802.
Fajans SS, Bell GI. MODY: history, genetics, pathophysiology, and clinical decision making. Diabetes Care. 2011;34:1878–84.
Chen J, Sun M, Adeyemo A, Pirie F, Carstensen T, Pomilla C, et al. Genome-wide association study of type 2 diabetes in Africa. Diabetologia. 2019;62:1204–11.
Qi Q, Stilp AM, Sofer T, Moon J-Y, Hidalgo B, Szpiro AA, et al. Genetics of type 2 diabetes in US Hispanic/Latino individuals: results from the Hispanic Community Health Study/Study of Latinos (HCHS/SOL). Diabetes. 2017;66:1419–25.
Spracklen CN, Horikoshi M, Kim YJ, Lin K, Bragg F, Moon S, et al. Identification of type 2 diabetes loci in 433,540 East Asian individuals. Nature. 2020;582:240–5.
Mahajan A, Wessel J, Willems SM, Zhao W, Robertson NR, Chu AY, et al. Refining the accuracy of validated target identification through coding variant fine-mapping in type 2 diabetes. Nat Genet. 2018;50:559–71.
Mahajan A, Go MJ, Zhang W, Below JE, Gaulton KJ, Ferreira T, et al. Genome-wide trans-ancestry meta-analysis provides insight into the genetic architecture of type 2 diabetes susceptibility. Nat Genet. 2014;46:234–44.
Mahajan A, Sim X, Zhang W, Below JE, Kitajima H, Speidel L, et al. 303-OR: ADA presidents’ select abstract: transethnic association study of type 2 diabetes in more than a million individuals. Diabetes. 2019;68.
Vujkovic M, Keaton JM, Lynch JA, Miller DR, Zhou J, Tcheandjieu C, Huffman JE, et al. Discovery of 318 new risk loci for type 2 diabetes and related vascular outcomes among 1.4 million participants in a multi-ancestry meta-analysis. Nat Genet. 2020;52:680–91.
Langenberg C, Lotta LA. Genomic insights into the causes of type 2 diabetes. Lancet. 2018;391:2463–74.
Flannick J, Mercader JM, Fuchsberger C, Udler MS, Mahajan A, Wessel J, et al. Exome sequencing of 20,791 cases of type 2 diabetes and 24,440 controls. Nature. 2019;570:71–6.
Dwivedi OP, Lehtovirta M, Hastoy B, Chandra V, Krentz NA, Kleiner S, et al. Loss of ZnT8 function protects against diabetes by enhanced insulin secretion. Nat Genet. 2019;51:1596–606.
Fuchsberger C, Flannick J, Teslovich TM, Mahajan A, Agarwala V, Gaulton KJ, et al. The genetic architecture of type 2 diabetes. Nature. 2016;536:41–7.
Jambaljav B, Tanaka D, Nagashima K, Harashima SI, Harada N, Harada T, et al. Whole-exome sequencing in a Japanese family with highly aggregated diabetes identifies a candidate susceptibility mutation in ADAMTSL3. Diabetes Res Clin Pract. 2018;135:143–9.
Johansson S, Irgens H, Chudasama KK, Molnes J, Aerts J, Roque FS, et al. Exome sequencing and genetic testing for MODY. PLoS One. 2012;7:e38050.
Yki-Jarvinen H, Koivisto VA. Natural course of insulin resistance in type I diabetes. N Engl J Med. 1986;315:224–30.
Cleland SJ, Fisher BM, Colhoun HM, Sattar N, Petrie JR. Insulin resistance in type 1 diabetes: what is ‘double diabetes’ and what are the risks? Diabetologia. 2013;56:1462–70.
Morris AP, Voight BF, Teslovich TM, Ferreira T, Segre AV, Steinthorsdottir V, et al. Large-scale association analysis provides insights into the genetic architecture and pathophysiology of type 2 diabetes. Nat Genet. 2012;44:981–90.
Scott RA, Lagou V, Welch RP, Wheeler E, Montasser ME, Luan J, et al. Large-scale association analyses identify new loci influencing glycemic traits and provide insight into the underlying biological pathways. Nat Genet. 2012;44:991–1005.
Gaulton KJ, Ferreira T, Lee Y, Raimondo A, Magi R, Reschen ME, et al. Genetic fine mapping and genomic annotation defines causal mechanisms at type 2 diabetes susceptibility loci. Nat Genet. 2015;47:1415–25.
Portha B, Chavey A, Movassat J. Early-life origins of type 2 diabetes: fetal programming of the beta-cell mass. Exp Diabetes Res. 2011;2011:105076.
Matveyenko AV, Butler PC. Relationship between beta-cell mass and diabetes onset. Diabetes Obes Metab. 2018;10:23–31.
Campbell-Thompson M, Fu A, Kaddis JS, Wasserfall C, Schatz DA, Pugliese A, et al. Insulitis and beta-cell mass in the natural history of type 1 diabetes. Diabetes. 2016;65:719–31.
Ferrannini E, Gastaldelli A, Miyazaki Y, Matsuda M, Mari A, DeFronzo RA. beta-Cell function in subjects spanning the range from normal glucose tolerance to overt diabetes: a new analysis. J Clin Endocrinol Metab. 2005;90:493–500.
Skyler JS, Bakris GL, Bonifacio E, Darsow T, Eckel RH, Groop L, et al. Differentiation of diabetes by pathophysiology, natural history, and prognosis. Diabetes. 2017;66:241–55.
Christensen AA, Gannon M. The beta cell in type 2 diabetes. Curr Diab Rep. 2019;19:81.
Halban PA, Polonsky KS, Bowden DW, Hawkins MA, Ling C, Mather KJ, et al. beta-cell failure in type 2 diabetes: postulated mechanisms and prospects for prevention and treatment. Diabetes Care. 2014;37:1751–8.
Yamamoto WR, Bone RN, Sohn P, Syed F, Reissaus CA, Mosley AL, et al. Endoplasmic reticulum stress alters ryanodine receptor function in the murine pancreatic beta cell. J Biol Chem. 2019;294:168–81.
Esser N, Legrand-Poels S, Piette J, Scheen AJ, Paquot N. Inflammation as a link between obesity, metabolic syndrome and type 2 diabetes. Diabetes Res Clin Pract. 2014;105:141–50.
Pradhan AD, Manson JE, Rifai N, Buring JE, Ridker PM. C-reactive protein, interleukin 6, and risk of developing type 2 diabetes mellitus. JAMA. 2001;286:327–34.
Bunney PE, Zink AN, Holm AA, Billington CJ, Kotz CM. Orexin activation counteracts decreases in nonexercise activity thermogenesis (NEAT) caused by high-fat diet. Physiol Behav. 2017;176:139–48.
Venkatasamy VV, Pericherla S, Manthuruthil S, Mishra S, Hanno R. Effect of physical activity on insulin resistance, inflammation and oxidative stress in diabetes mellitus. J Clin Diagn Res. 2013;7:1764–6.
Sircana A, Framarin L, Leone N, Berrutti M, Castellino F, Parente R, et al. Altered gut microbiota in type 2 diabetes: just a coincidence? Curr Diab Rep. 2018;18:98.
Wang R, Tang R, Li B, Ma X, Schnabl B, Tilg H. Gut microbiome, liver immunology, and liver diseases. Cell Mol Immunol. 2021;18:4–17.
Crittenden S, Goepp M, Pollock J, Robb CT, Smyth DJ, Zhou Y, et al. Prostaglandin E(2) promotes intestinal inflammation via inhibiting microbiota-dependent regulatory T cells. Sci Adv. 2021;7.
Han Q, Wang J, Li W, Chen ZJ, Du Y. Androgen-induced gut dysbiosis disrupts glucolipid metabolism and endocrinal functions in polycystic ovary syndrome. Microbiome. 2021;9:101.
Zhou Z, Sun B, Yu D, Zhu C. Gut microbiota: an important player in type 2 diabetes mellitus. Front Cell Infect Microbiol. 2022;12:834485.
Takagi T, Naito Y, Kashiwagi S, Uchiyama K, Mizushima K, Kamada K, et al. Changes in the gut microbiota are associated with hypertension, hyperlipidemia, and type 2 diabetes mellitus in Japanese subjects. Nutrients. 2020;12.
Wang TY, Zhang XQ, Chen AL, Zhang J, Lv BH, Ma MH, et al. A comparative study of microbial community and functions of type 2 diabetes mellitus patients with obesity and healthy people. Appl Microbiol Biotechnol. 2020;104:7143–53.
Yang K, Niu J, Zuo T, Sun Y, Xu Z, Tang W, et al. Alterations in the gut virome in obesity and type 2 diabetes mellitus. Gastroenterology. 2021;161:1257–69.e13.
Al Bataineh MT, Dash NR, Bel Lassen P, Banimfreg BH, Nada AM, Belda E, et al. Revealing links between gut microbiome and its fungal community in Type 2 Diabetes Mellitus among Emirati subjects: a pilot study. Sci Rep. 2020;10:9624.
Federation I IDF Diabetes Atlas Eighth edition 2019. International Diabetes Federation. IDF Diabetes Atlas, 9th edn. Brussels. Belgium: International Diabetes Federation 2019.
Forouhi NG, Wareham N. Epidemiology of diabetes. Medicine. 2019;47:22–7.
Fitzmaurice C, Abate D, Abbasi N. Global Burden of Disease Cancer Collaboration Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 29 cancer groups, 1990 to 2017: a systemic analysis for the Global Burden of Disease Study. Jama Oncol. 2020;6:444.
Saeedi P, Petersohn I, Salpea P, et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res Clin Pract. 2019;157:107843.
Herman WH, Ye W, Griffin SJ, Simmons RK, Davies MJ, Khunti K, et al. Early detection and treatment of type 2 diabetes reduce cardiovascular morbidity and mortality: a simulation of the results of the Anglo-Danish-Dutch Study of Intensive Treatment in People With Screen-Detected Diabetes in Primary Care (ADDITION-Europe). Diabetes Care. 2015;38:1449–55.
Chong S, Ding D, Byun R, Comino E, Bauman A, Jalaludin B. Lifestyle changes after a diagnosis of type 2 diabetes. diabetes spectr. Diabetes Spectr. 2017;30:43–50.
Cotter AP, Durant N, Agne AA, Cherrington A. Internet interventions to support lifestyle modification for diabetes management: a systematic review of the evidence. J Diabetes Complications. 2014;28:243–51.
Contreras I, Vehi J. Artificial intelligence for diabetes management and decision support: literature review. J Med Internet Res. 2018;20:e10775.
Ellahham S. Artificial intelligence: the future for diabetes care. Am J Med. 2020;133:895–900.
Buch V, Varughese G, Maruthappu M. Artificial intelligence in diabetes care. Diabetic Med. 2018;35:495–7.
Food U Drug Administration (FDA). FDA permits marketing of artificial intelligence-based device to detect certain diabetes-related eye problems. 2018.
Association AD. 11. Microvascular complications and foot care: standards of medical care in diabetes—2020. Diabetes Care. 2020;43:S135–S51.
Ellahham S, Ellahham N. Use of artificial intelligence for improving patient flow and healthcare delivery. J Comp Sci Syst Biol. 2019;12:2.
Bellemo V, Lim G, Rim TH, Tan GS, Cheung CY, Sadda S, et al. Artificial intelligence screening for diabetic retinopathy: the real-world emerging application. Curr Diab Rep. 2019;19:1–12.
Dunbar CE, High KA, Joung JK, Kohn DB, Ozawa K, Sadelain M. Gene therapy comes of age. Science. 2018;359:eaan4672.
Tsokos GC, Nepom GT. Gene therapy in the treatment of autoimmune diseases. J Clin Invest. 2000;106:181–3.
Chellappan DK, Yap WS, Na BAS, Gupta G, Dua K. Current therapies and targets for type 2 diabetes mellitus. Panminerva Med. 2018;60:117–31.
Bakay M, Pandey R, Hakonarson H. Genes involved in type 1 diabetes: an update. Genes. 2013;4:499–521.
Chan L, Fujimiya M, Kojima H. In vivo gene therapy for diabetes mellitus. Trends Mol Med. 2003;9:430–5.
Zalzman M, Gupta S, Giri RK, et al. Reversal of hyperglycemia in mice by using human expandable insulin-producing cells differentiated from fetal liver progenitor cells. Proc Natl Acad Sci USA. 2003;100:7253–8.
Kwak SH, Park KS. Recent progress in genetic and epigenetic research on type 2 diabetes. Exp Mol Med. 2016;48:e220.
Abderrazak A, El Hadri K, Bosc E, Blondeau B, Slimane M-N, Büchele B, et al. Inhibition of the inflammasome NLRP3 by arglabin attenuates inflammation, protects pancreatic β-cells from apoptosis, and prevents type 2 diabetes mellitus development in ApoE2Ki mice on a chronic high-fat diet. J Pharmacol Exp Ther. 2016;357:487–94.
O'Doherty RM, Jensen PB, Anderson P, et al. Activation of direct and indirect pathways of glycogen synthesis by hepatic overexpression of protein targeting to glycogen. J Clin Invest. 2000;105:479–88.
Newgard CB, Brady MJ, O'Doherty RM, Saltiel AR. Organizing glucose disposal: emerging roles of the glycogen targeting subunits of protein phosphatase-1. Diabetes. 2000;49:1967–77.
Dong H, SLJTiE Woo. Hepatic insulin production for type 1 diabetes. Trends Endocrinol Metab. 2001;12:441–6.
Auricchio A, Gao GP, Yu QC, et al. Constitutive and regulated expression of processed insulin following in vivo hepatic gene transfer. Gene Ther. 2002;9:963–71.
Kojima H, Fujimiya M, Matsumura K, et al. NeuroD-betacellulin gene therapy induces islet neogenesis in the liver and reverses diabetes in mice. Nat Med. 2003;9:596–603.
Pastors JG, Warshaw H, Daly A, Franz M, Kulkarni K. The evidence for the effectiveness of medical nutrition therapy in diabetes management. Diabetes Care. 2002;25:608–13.
Tiwari P. Recent trends in therapeutic approaches for diabetes management: a comprehensive update. J Diabetes Res. 2015;2015.
Association AD. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2014;37:S81–S90.
Evert AB, Boucher JL, Cypress M, Dunbar SA, Franz MJ, Mayer-Davis EJ, et al. Nutrition therapy recommendations for the management of adults with diabetes. Diabetes Care. 2014;37:S120–S43.
Bantle JP, Wylie-Rosett J, Albright AL, Apovian CM, Clark NG, Franz MJ, et al. Nutrition recommendations and interventions for diabetes: a position statement of the American Diabetes Association. Diabetes Care. 2008;31:S61–S78.
DiSanto RM, Subramanian V, Gu Z. Recent advances in nanotechnology for diabetes treatment. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2015;7:548–64.
Aloke C, Egwu CO, Aja PM, Obasi NA, Chukwu J, Akumadu BO, et al. Current advances in the management of diabetes mellitus. Biomedicines. 2022;10:2436.
Tamborlane W, Beck R, Bode B, Buckingham B, Chase H, Clemons R. Juvenile Diabetes Research Foundation Continuous Glucose Monitoring Study Group et al. Continuous glucose monitoring and intensive treatment of type 1 diabetes. N Engl J Med.2008;359:1464–76.
Edelman SV, Argento NB, Pettus J, Hirsch IB. Clinical implications of real-time and intermittently scanned continuous glucose monitoring. Diabetes Care. 2018;41:2265–74.
Veiseh O, Tang BC, Whitehead KA, Anderson DG, Langer R. Managing diabetes with nanomedicine: challenges and opportunities. Nat Rev Drug Discov. 2015;14:45–57.
Scognamiglio V. Nanotechnology in glucose monitoring: advances and challenges in the last 10 years. Biosens Bioelectron. 2013;47:12–25.
Grunberger G. The need for better insulin therapy. Diabetes Obes Metab. 2013;15:1–5.
Lagopati N, Pavlatou E. Nanotechnology in diabetes management. Interv Obes Diabetes. 2021;5:419–24.
Lemmerman LR, Das D, Higuita-Castro N, Mirmira RG, Gallego-Perez D. Nanomedicine-based strategies for diabetes: diagnostics, monitoring, and treatment. Trends Endocrinol Metab. 2020;31:448–58.
McCall MD, Toso C, Baetge EE, Shapiro AJ. Are stem cells a cure for diabetes? Clin Sci. 2010;118:87–97.
Abdulazeez SS. Diabetes treatment: a rapid review of the current and future scope of stem cell research. Saudi Pharm J. 2015;23:333–40.
Bonner-Weir S, Baxter LA, Schuppin GT, Smith FE. A second pathway for regeneration of adult exocrine and endocrine pancreas: a possible recapitulation of embryonic development. Diabetes. 1993;42:1715–20.
Bonner-Weir S, Taneja M, Weir GC, Tatarkiewicz K, Song K-H, Sharma A, et al. In vitro cultivation of human islets from expanded ductal tissue. Proc Natl Acad Sci. 2000;97:7999–8004.
Gao R, Ustinov J, Pulkkinen M-A, Lundin K, Korsgren O, Otonkoski T. Characterization of endocrine progenitor cells and critical factors for their differentiation in human adult pancreatic cell culture. Diabetes. 2003;52:2007–15.
Ianus A, Holz GG, Theise ND, Hussain MA. In vivo derivation of glucose-competent pancreatic endocrine cells from bone marrow without evidence of cell fusion. J Clin Invest. 2003;111:843–50.
Hess D, Li L, Martin M, Sakano S, Hill D, Strutt B, et al. Bone marrow–derived stem cells initiate pancreatic regeneration. Nat Biotechnol. 2003;21:763–70.
Shah RV, Goldfine AB. Statins and risk of new-onset diabetes mellitus. Circulation. 2012;126:e282–e4.
Chen Y-H, Feng B, Chen Z-W. Statins for primary prevention of cardiovascular and cerebrovascular events in diabetic patients without established cardiovascular diseases: a meta-analysis. Exp Clin Endocrinol Diabetes. 2012;120:116–20.
Buse J. Statin treatment in diabetes mellitus. Clin Diabetes. 2003;21:168–72.
Drummond RS, Lyall M, McKnight J. Statins should be routinely prescribed in all adults with diabetes. Pract Diabetes Int. 2010;27:404–6a.
Food U, Administration D FDA approves novel, dual-targeted treatment for type 2 diabetes. FDA News Release. 2022.
Bertsch T. An introduction to tirzepatide. Clin Diabetes. 2022;40:371–2.
Bailey CJ, Flatt PR, Conlon JM. An update on peptide-based therapies for type 2 diabetes and obesity. Peptides. 2023;161:170939.
Nauck MA, Mirna AEA, Quast DR. Meta-analysis of head-to-head clinical trials comparing incretin-based glucose-lowering medications and basal insulin: An update including recently developed glucagon-like peptide-1 (GLP-1) receptor agonists and the glucose-dependent insulinotropic polypeptide/GLP-1 receptor co-agonist tirzepatide. Diabetes Obes Metab. 2023;25:1361–71.
Madsbad S, Holst JJ. Cardiovascular effects of incretins: focus on glucagon-like peptide-1 receptor agonists. Cardiovasc Res. 2023;119:886–904.
Piemonti L, Keymeulen B, Gillard P, Linn T, Bosi E, Rose L, et al. Ladarixin, an inhibitor of the interleukin‐8 receptors CXCR1 and CXCR2, in new‐onset type 1 diabetes: a multicentre, randomized, double‐blind, placebo‐controlled trial. Diabetes Obes Metab. 2022;24:1840.
Kawai T, Sun B, Yoshino H, Feng D, Suzuki Y, Fukazawa M, et al. Structural basis for GLP-1 receptor activation by LY3502970, an orally active nonpeptide agonist. Proc Natl Acad Sci. 2020;117:29959–67.
Fesenko I, Azarkina R, Kirov I, Kniazev A, Filippova A, Grafskaia E, et al. Phytohormone treatment induces generation of cryptic peptides with antimicrobial activity in the Moss Physcomitrella patens. BMC Plant Biol. 2019;19:9.
Karami Z, Akbari-Adergani B. Bioactive food derived peptides: a review on correlation between structure of bioactive peptides and their functional properties. J Food Sci Technol. 2019;56:535–47.
Akbarian M, Khani A, Eghbalpour S, Uversky VN. Bioactive peptides: synthesis, sources, applications, and proposed mechanisms of action. Int J Mol Sci. 2022;23.
Qiao Q, Chen L, Li X, Lu X, Xu Q. Roles of dietary bioactive peptides in redox balance and metabolic disorders. Oxid Med Cell Longev. 2021;2021:5582245.
Antony P, Vijayan R. Bioactive peptides as potential nutraceuticals for diabetes therapy: a comprehensive review. Int J Mol Sci. 2021;22.
Muttenthaler M, King GF, Adams DJ, Alewood PF. Trends in peptide drug discovery. Nat Rev Drug Discov. 2021;20:309–25.
Yan J, Zhao J, Yang R, Zhao W. Bioactive peptides with antidiabetic properties: a review. Int J Food Sci Technol. 2019;54:1909–19.
Singh AK. Dipeptidyl peptidase-4 inhibitors: novel mechanism of actions. Indian J Endocrinol Metab. 2014;18:753–9.
Wang L, Wang N, Zhang W, Cheng X, Yan Z, Shao G, et al. Therapeutic peptides: current applications and future directions. Signal Transduct Target Ther. 2022;7:48.
Zaky AA, Simal-Gandara J, Eun JB, Shim JH, Abd El-Aty AM. Bioactivities, applications, safety, and health benefits of bioactive peptides from food and by-products: a review. Front Nutr. 2021;8:815640.
Furman BL. The development of Byetta (exenatide) from the venom of the Gila monster as an anti-diabetic agent. Toxicon. 2012;59:464–71.
Wajcberg E, Amarah A. Liraglutide in the management of type 2 diabetes. Drug Des Devel Ther. 2010;4:279–90.
Wang Z, Zhang X. Isolation and identification of anti-proliferative peptides from Spirulina platensis using three-step hydrolysis. J Sci Food Agric. 2017;97:918–22.
Zambrowicz A, Eckert E, Pokora M, Bobak Ł, Dąbrowska A, Szołtysik M, et al. Antioxidant and antidiabetic activities of peptides isolated from a hydrolysate of an egg-yolk protein by-product prepared with a proteinase from Asian pumpkin (Cucurbita ficifolia). RSC Adv. 2015;5:10460–7.
Wang R, Zhao H, Pan X, Orfila C, Lu W, Ma Y. Preparation of bioactive peptides with antidiabetic, antihypertensive, and antioxidant activities and identification of alpha-glucosidase inhibitory peptides from soy protein. Food Sci Nutr. 2019;7:1848–56.
Karimi A, Azizi MH, Ahmadi Gavlighi H. Frationation of hydrolysate from corn germ protein by ultrafiltration: In vitro antidiabetic and antioxidant activity. Food Sci Nutr. 2020;8:2395–405.
Ktari N, Salem RBS-B, Bkhairia I, Slima SB, Nasri R, Salah RB, et al. Functional properties and biological activities of peptides from zebra blenny protein hydrolysates fractionated using ultrafiltration. Food Biosci. 2020;34:100539.
Dale HF, Jensen C, Hausken T, et al. Effect of a cod protein hydrolysate on postprandial glucose metabolism in healthy subjects: a double-blind cross-over trial [published correction appears in J Nutr Sci. 2019 Jan 18;8:e1]. J Nutr Sci. 2018;7:e33.
Wang T, Zheng L, Zhao T, Zhang Q, Liu Z, Liu X, et al. Anti-diabetic effects of sea cucumber (Holothuria nobilis) hydrolysates in streptozotocin and high-fat-diet induced diabetic rats via activating the PI3K/Akt pathway. J Funct Foods. 2020;75.
Godinho R, Mega C, Teixeira-de-Lemos E, Carvalho E, Teixeira F, Fernandes R, et al. The place of dipeptidyl peptidase-4 inhibitors in type 2 diabetes therapeutics: a “me too” or “the special one” antidiabetic class? J Diabetes Res. 2015;2015:806979.
Richter B, Bandeira-Echtler E, Bergerhoff K, Lerch C. Emerging role of dipeptidyl peptidase-4 inhibitors in the management of type 2 diabetes. Vasc Health Risk Manag. 2008;4:753–68.
Jin R, Teng X, Shang J, Wang D, Liu N. Identification of novel DPP-IV inhibitory peptides from Atlantic salmon (Salmo salar) skin. Food Res Int. 2020;133:109161.
Gao J, Gong H, Mao X. Dipeptidyl peptidase-IV inhibitory activity and related molecular mechanism of bovine alpha-lactalbumin-derived peptides. Molecules. 2020;25.
Gu H, Gao J, Shen Q, Gao D, Wang Q, Tangyu M, et al. Dipeptidyl peptidase-IV inhibitory activity of millet protein peptides and the related mechanisms revealed by molecular docking. LWT. 2021;138:110587.
Wang J, Wu T, Fang L, Liu C, Liu X, Li H, et al. Anti-diabetic effect by walnut (Juglans mandshurica Maxim.)-derived peptide LPLLR through inhibiting α-glucosidase and α-amylase, and alleviating insulin resistance of hepatic HepG2 cells. J Funct Foods. 2020;69.
Depta J, Małkowska P, Wysokińska M, Todorska K, Sierawska O, Hrynkiewicz R, et al. Therapeutic role of antimicrobial peptides in diabetes mellitus. Biologics. 2022;2:92–106.
Moretta A, Scieuzo C, Petrone AM, Salvia R, Manniello MD, Franco A, et al. Antimicrobial peptides: a new hope in biomedical and pharmaceutical fields. Front Cell Infect Microbiol. 2021;11:668632.
Hirsch JG. Phagocytin: a bactericidal substance from polymorphonuclear leucocytes. J Exp Med. 1956;103:589–611.
Bahar AA, Ren D. Antimicrobial peptides. Pharmaceuticals (Basel). 2013;6:1543–75.
Miller A, Matera-Witkiewicz A, Mikolajczyk A, Wieczorek R, Rowinska-Zyrek M. Chemical “butterfly effect” explaining the coordination chemistry and antimicrobial properties of clavanin complexes. Inorg Chem. 2021;60:12730–4.
Zhang QY, Yan ZB, Meng YM, Hong XY, Shao G, Ma JJ, et al. Antimicrobial peptides: mechanism of action, activity and clinical potential. Mil Med Res. 2021;8:48.
van Harten RM, van Woudenbergh E, van Dijk A, Haagsman HP. Cathelicidins: immunomodulatory antimicrobials. Vaccines (Basel). 2018;6.
Nagaoka I, Tamura H, Reich J. Therapeutic potential of cathelicidin peptide LL-37, an Antimicrobial agent, in a murine sepsis model. Int J Mol Sci. 2020;21.
Contreras G, Shirdel I, Braun MS, Wink M. Defensins: transcriptional regulation and function beyond antimicrobial activity. Dev Comp Immunol. 2020;104:103556.
Jenssen H, Hamill P, Hancock RE. Peptide antimicrobial agents. Clin Microbiol Rev. 2006;19:491–511.
Miani M, Le Naour J, Waeckel-Enee E, Verma SC, Straube M, Emond P, et al. Gut microbiota-stimulated innate lymphoid cells support beta-defensin 14expression in pancreatic endocrine cells, preventing autoimmune diabetes. Cell Metab. 2018;28:557–72.e6.
Tsai YW, Dong JL, Jian YJ, Fu SH, Chien MW, Liu YW, et al. Gut Microbiota-modulated metabolomic profiling shapes the etiology and pathogenesis of autoimmune diseases. Microorganisms. 2021;9.
Coughlan KA, Valentine RJ, Ruderman NB, Saha AK. AMPK activation: a therapeutic target for type 2 diabetes? Diabetes Metab Syndr Obes. 2014;7:241–53.
Zainab AJAA, Ashish N, Ragnath V. Salivary levels of antimicrobial peptides in chronic periodontitis patients with type 2 diabetes. J Int Acad Periodontol. 2019;21:36–44.
Soltaninejad H, Zare-Zardini H, Ordooei M, Ghelmani Y, Ghadiri-Anari A, Mojahedi S, et al. Antimicrobial peptides from amphibian innate immune system as potent antidiabetic agents: a literature review and bioinformatics analysis. J Diabetes Res. 2021;2021:2894722.
Musale V, Moffett RC, Owolabi B, Conlon JM, Flatt PR, Abdel-Wahab YHA. Mechanisms of action of the antidiabetic peptide [S4K]CPF-AM1 in db/db mice. J Mol Endocrinol. 2021;66:115–28.
Ramadhan AH, Nawas T, Zhang X, Pembe WM, Xia W, Xu Y. Purification and identification of a novel antidiabetic peptide from Chinese giant salamander (Andrias davidianus) protein hydrolysate against α-amylase and α-glucosidase. Int J Food Properties. 2017;20:S3360–S72.
Nishikawa T, Araki E. Investigation of a novel mechanism of diabetic complications: impacts of mitochondrial reactive oxygen species. Rinsho Byori. 2008;56:712–9.
Rolo AP, Palmeira CM. Diabetes and mitochondrial function: role of hyperglycemia and oxidative stress. Toxicol Appl Pharmacol. 2006;212:167–78.
Bonnefont-Rousselot D. Glucose and reactive oxygen species. Curr Opin Clin Nutr Metab Care. 2002;5:561–8.
Erbguth F. Diabetes and the central nervous system. Nervenarzt. 2017;88:675–90.
Zarse K, Ristow M. A mitochondrially encoded hormone ameliorates obesity and insulin resistance. Cell Metab. 2015;21:355–6.
Onyango AN. Cellular stresses and stress responses in the pathogenesis of insulin resistance. Oxid Med Cell Longev. 2018;2018:4321714.
Martin-Montalvo A, Mercken EM, Mitchell SJ, Palacios HH, Mote PL, Scheibye-Knudsen M, et al. Metformin improves healthspan and lifespan in mice. Nat Commun. 2013;4:2192.
Ikonen M, Liu B, Hashimoto Y, Ma L, Lee KW, Niikura T, et al. Interaction between the Alzheimer’s survival peptide humanin and insulin-like growth factor-binding protein 3 regulates cell survival and apoptosis. Proc Natl Acad Sci USA. 2003;100:13042–7.
Kwon C, Sun JL, Jeong JH, Jung TW. Humanin attenuates palmitate-induced hepatic lipid accumulation and insulin resistance via AMPK-mediated suppression of the mTOR pathway. Biochem Biophys Res Commun. 2020;526:539–45.
Kuliawat R, Klein L, Gong Z, Nicoletta-Gentile M, Nemkal A, Cui L, et al. Potent humanin analog increases glucose-stimulated insulin secretion through enhanced metabolism in the beta cell. FASEB J. 2013;27:4890–8.
Cobb LJ, Lee C, Xiao J, Yen K, Wong RG, Nakamura HK, et al. Naturally occurring mitochondrial-derived peptides are age-dependent regulators of apoptosis, insulin sensitivity, and inflammatory markers. Aging (Albany NY). 2016;8:796–809.
Lee C, Zeng J, Drew BG, Sallam T, Martin-Montalvo A, Wan J, et al. The mitochondrial-derived peptide MOTS-c promotes metabolic homeostasis and reduces obesity and insulin resistance. Cell Metab. 2015;21:443–54.
Lu H, Wei M, Zhai Y, Li Q, Ye Z, Wang L, et al. MOTS-c peptide regulates adipose homeostasis to prevent ovariectomy-induced metabolic dysfunction. J Mol Med (Berl). 2019;97:473–85.
Zhai D, Ye Z, Jiang Y, Xu C, Ruan B, Yang Y, et al. MOTS-c peptide increases survival and decreases bacterial load in mice infected with MRSA. Mol Immunol. 2017;92:151–60.
Bloomgarden ZT. Inflammation and insulin resistance. Diabetes Care. 2003;26:1619–23.
Simsek S. Angiotensin I-converting enzyme, dipeptidyl peptidase-IV, and α-glucosidase inhibitory potential of hazelnut meal protein hydrolysates. J Food Measurement Charact. 2021;15:4490–6.
Yu Z, Yin Y, Zhao W, Liu J, Chen F. Anti-diabetic activity peptides from albumin against α-glucosidase and α-amylase. Food Chem. 2012;135:2078–85.
Mudgil P, Kilari BP, Kamal H, Olalere OA, FitzGerald RJ, Gan C-Y, et al. Multifunctional bioactive peptides derived from quinoa protein hydrolysates: Inhibition of α-glucosidase, dipeptidyl peptidase-IV and angiotensin I converting enzymes. J Cereal Sci. 2020;96:103130.
Vilcacundo R, Martínez-Villaluenga C, Hernández-Ledesma B. Release of dipeptidyl peptidase IV, α-amylase and α-glucosidase inhibitory peptides from quinoa (Chenopodium quinoa Willd.) during in vitro simulated gastrointestinal digestion. J Funct Foods. 2017;35:531–9.
Fuentes LR, Richard C, Chen L. Sequential alcalase and flavourzyme treatment for preparation of α-amylase, α-glucosidase, and dipeptidyl peptidase (DPP)-IV inhibitory peptides from oat protein. J Funct Foods. 2021;87:104829.
Pathan S, Siddiqui RA. Nutritional composition and bioactive components in Quinoa (Chenopodium quinoa Willd.) greens: a review. Nutrients. 2022;14.
Salami M, Sadeghian Motahar SF, Ariaeenejad S, Sheykh Abdollahzadeh Mamaghani A, Kavousi K, Moosavi-Movahedi AA, et al. The novel homologue of the human alpha-glucosidase inhibited by the non-germinated and germinated quinoa protein hydrolysates after in vitro gastrointestinal digestion. J Food Biochem. 2022;46:e14030.
Luan F, Wang Z, Yang Y, Ji Y, Lv H, Han K, et al. Juglans mandshurica Maxim.: a review of its traditional usages, phytochemical constituents, and pharmacological properties. Front Pharmacol. 2020;11:569800.
Ghafoor K, Özcan MM, AL-Juhaımı F, Babıker EE, Sarker ZI, Ahmed IAM, et al. Nutritional composition, extraction, and utilization of wheat germ oil: a review. Eur J Lipid Sci Technol. 2017;119:1600160.
Liu W, Li H, Wen Y, Liu Y, Wang J, Sun B. Molecular mechanism for the alpha-glucosidase inhibitory effect of wheat germ peptides. J Agric Food Chem. 2021;69:15231–9.
Rasane P, Jha A, Sabikhi L, Kumar A, Unnikrishnan VS. Nutritional advantages of oats and opportunities for its processing as value added foods - a review. J Food Sci Technol. 2015;52:662–75.
Grundy MM, Fardet A, Tosh SM, Rich GT, Wilde PJ. Processing of oat: the impact on oat’s cholesterol lowering effect. Food Funct. 2018;9:1328–43.
Zhang Y, Wu F, He Z, Fang X, Liu X. Optimization and molecular mechanism of novel alpha-glucosidase inhibitory peptides derived from camellia seed cake through enzymatic hydrolysis. Foods. 2023;12.
Feng J, Ma YL, Sun P, Thakur K, Wang S, Zhang JG, et al. Purification and characterisation of α‐glucosidase inhibitory peptides from defatted camellia seed cake. Int J Food Sci Technol. 2021;56:138–47.
Brown R, Ware L, Tey SL. Effects of hazelnut consumption on cardiometabolic risk factors and acceptance: a systematic review. Int J Environ Res Public Health. 2022;19.
Gul K, Yousuf B, Singh AK, Singh P, Wani AA. Rice bran: nutritional values and its emerging potential for development of functional food—a review. Bioact Carbohydr Diet Fibre. 2015;6:24–30.
Uraipong C, Zhao J. In vitro digestion of rice bran proteins produces peptides with potent inhibitory effects on alpha-glucosidase and angiotensin I converting enzyme. J Sci Food Agric. 2018;98:758–66.
Tan SP, Kha TC, Parks SE, Roach PD. Bitter melon (Momordica charantia L.) bioactive composition and health benefits: a review. Food Rev Int. 2016;32:181–202.
Poovitha S, Parani M. In vitro and in vivo alpha-amylase and alpha-glucosidase inhibiting activities of the protein extracts from two varieties of bitter gourd (Momordica charantia L.). BMC Complement Altern Med. 2016;16:185.
Kumar P, Mahato DK, Kamle M, Borah R, Sharma B, Pandhi S, et al. Pharmacological properties, therapeutic potential, and legal status of Cannabis sativa L.: an overview. Phytother Res. 2021;35:6010–29.
Ren Y, Liang K, Jin Y, Zhang M, Chen Y, Wu H, et al. Identification and characterization of two novel α-glucosidase inhibitory oligopeptides from hemp (Cannabis sativa L.) seed protein. J Funct Foods. 2016;26:439–50.
Telapolu S, Kalachavedu M, Punnoose AM, Bilikere DMD-1. a poly herbal formulation indicated in diabetes mellitus ameliorates glucose uptake and inhibits adipogenesis - an in vitro study. BMC Complement Altern Med. 2018;18:113.
Butala MA, Kukkupuni SK, Venkatasubramanian P, Vishnuprasad CN. An ayurvedic anti-diabetic formulation made from Curcuma longa L. and Emblica officinalis L. inhibits α-amylase, α-glucosidase, and starch digestion, in vitro. Starch Stärke. 2018;70:1700182.
Panda V, Deshmukh A, Singh S, Shah T, Hingorani L. An ayurvedic formulation of Emblica officinalis and Curcuma longa alleviates insulin resistance in diabetic rats: involvement of curcuminoids and polyphenolics. J Ayurveda Integr Med. 2021;12:506–13.
Mehrzadi S, Tavakolifar B, Huseini HF, Mosavat SH, Heydari M. The effects of Boswellia serrata gum resin on the blood glucose and lipid profile of diabetic patients: a double-blind randomized placebo-controlled clinical trial. J Evid Based Integr Med. 2018;23:2515690X18772728.
Ahmed A, Zeng G, Azhar M, Lin H, Zhang M, Wang F, et al. Jiawei Shengmai San herbal formula ameliorates diabetic associate cognitive decline by modulating AKT and CREB in rats. Phytother Res. 2020;34:3249–61.
Yella SST, Kumar RN, Ayyanna C, Varghese AM, Amaravathi P, Vangoori Y. The combined effect of Trigonella foenum seeds and Coriandrum sativum leaf extracts in alloxan-induced diabetes mellitus wistar albino rats. Bioinformation. 2019;15:716–22.
Mehrzadi S, Mirzaei R, Heydari M, Sasani M, Yaqoobvand B, Huseini HF. Efficacy and safety of a traditional herbal combination in patients with type II diabetes mellitus: a randomized controlled trial. J Diet. 2021;18:31–43.
Wasana KGP, Attanayake AP, Weerarathna TP, Jayatilaka K. Efficacy and safety of a herbal drug of Coccinia grandis (Linn.) Voigt in patients with type 2 diabetes mellitus: a double blind randomized placebo controlled clinical trial. Phytomedicine. 2021;81:153431.
Wang H, Tan H, Zhan W, Song L, Zhang D, Chen X, et al. Molecular mechanism of Fufang Zhenzhu Tiaozhi capsule in the treatment of type 2 diabetes mellitus with nonalcoholic fatty liver disease based on network pharmacology and validation in minipigs. J Ethnopharmacol. 2021;274:114056.
Perez Gutierrez RM, Martinez Jeronimo FF, Contreras Soto JG, Muniz Ramirez A, Estrella, Mendoza MF. Optimization of ultrasonic-assisted extraction of polyphenols from the polyherbal formulation of Cinnamomum verum, Origanum majorana, and Origanum vulgare and their anti-diabetic capacity in zebrafish (Danio rerio). Heliyon. 2022;8:e08682.
Naik A, Adeyemi SB, Vyas B, Krishnamurthy R. Effect of co-administration of metformin and extracts of Costus pictus D.Don leaves on alloxan-induced diabetes in rats. J Tradit Complement Med. 2022;12:269–80.
Kumar A, Negi AS, Chauhan A, Semwal R, Kumar R, Semwal RB, et al. Formulation and evaluation of SGLT2 inhibitory effect of a polyherbal mixture inspired from Ayurvedic system of medicine. J Tradit Complement Med. 2022;12:477–87.
Majd FS, Talebi SS, Ahmad Abadi AN, Poorolajal J, Dastan D. Efficacy of a standardized herbal product from Pistacia atlantica subsp. Kurdica in type 2 diabetic patients with hyperlipidemia: a triple-blind randomized clinical trial. Complement Ther Clin Pract. 2022;48:101613.
Nguyen TT, Le QT, Hoang DT, et al. Massively parallel sequencing uncovered disease-associated variant spectra of glucose-6-phosphate dehydrogenase deficiency, phenylketonuria and galactosemia in Vietnamese pregnant women. Mol Genet Genomic Med. 2022;10:e1959.
Klak M, Gomółka M, Kowalska P, et al. Type 1 diabetes: genes associated with disease development. Cent Eur J Immunol. 2020;45:439–53.
Ahlqvist E, Ahluwalia TS, Groop L. Genetics of type 2 diabetes. Clin Chem. 2011;57:241–54.
Rafique I, Mir A, Saqib MAN, Naeem M, Marchand L, Polychronakos C. Causal variants in Maturity Onset Diabetes of the Young (MODY) - A systematic review. BMC Endocr Disord. 2021;21:223.
Huang SL, Jao CL, Ho KP, Hsu KC. Dipeptidyl-peptidase IV inhibitory activity of peptides derived from tuna cooking juice hydrolysates. Peptides. 2012;35:114–21.
Nongonierma AB, FitzGerald RJ. Inhibition of dipeptidyl peptidase IV (DPP-IV) by proline containing casein-derived peptides. J Funct Foods. 2013;5:1909–17.
Harnedy PA, Parthsarathy V, McLaughlin CM, et al. Atlantic salmon (Salmo salar) co-product-derived protein hydrolysates: A source of antidiabetic peptides. Food Res Int. 2018;106:598–606.
Zhang Y, Chen R, Chen X, Zeng Z, Ma H, Chen S. Dipeptidyl peptidase iv-inhibitory peptides derived from silver carp (hypophthalmichthys molitrix val.) proteins. J Agric Food Chem. 2016;64:831–9.
Valencia-Mejía E, Batista KA, Fernández JJA, Fernandes KF. Antihyperglycemic and hypoglycemic activity of naturally occurring peptides and protein hydrolysates from easy-to-cook and hard-to-cook beans (Phaseolus vulgaris L.). Food Res Int. 2019;121:238–46.
Acknowledgements
WC and SS are thankful to Indian Council of Medical Research (ICMR), New Delhi and Council of Scientific and Industrial Research (CSIR), New Delhi for providing senior research fellowship, respectively.
Author information
Authors and Affiliations
Contributions
Conceptualization: MAA, SS, and WC; writing—original draft: SS, WC, and MAA; writing—review and editing: SAA, MA, HA, MNA, and EAA; All authors were involved in drafting, reviewing and revising the final paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ansari, M.A., Chauhan, W., Shoaib, S. et al. Emerging therapeutic options in the management of diabetes: recent trends, challenges and future directions. Int J Obes 47, 1179–1199 (2023). https://doi.org/10.1038/s41366-023-01369-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41366-023-01369-3