Abstract
Background
FAM132b (myonectin) has been identified as a muscle-derived myokine with exercise and has hormone activity in circulation to regulate iron homeostasis and lipid metabolism via unknown receptors. Here, we aim to explore the potential of adeno-associated virus to deliver FAM132b in vivo to develop a gene therapy against obesity.
Methods
Adeno-associated virus AAV9 were engineered to induce overexpression of FAM132b with two mutations, A136T and P159A. Then, AAV9 was delivered into high-fat diet mice through tail vein, and glucose homeostasis and obesity development of mice were observed. Methods of structural biology were used to predict the action site or receptor of the FAM132b mutant.
Results
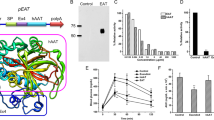
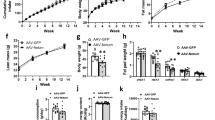
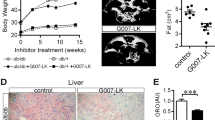
Treatment of high-fat diet-fed mice with AAV9 improved glucose intolerance and insulin resistance, and resulted in reductions in body weight, fat depot, and adipocyte size. Codon-optimized FAM132b (coFAM132b) reduced the glycemic response to epinephrine (EPI) in the whole body and increased the lipolytic response to EPI in adipose tissues. However, FAM132b knockdown by shRNA significantly increased the glycemic response to EPI in vivo and reduced adipocyte response to EPI and adipose tissue browning. Structural analysis predicted that the FAM132b mutant with A136T and P159A may form a weak bond with β2 adrenergic receptor (ADRB2) and may have more affinity for insulin and insulin-receptor complexes.
Conclusions
Our study underscores the potential of FAM132b gene therapy with codon optimization to treat obesity by modulating the adrenergic response and insulin action. Both structural biological analysis and in vivo experiments suggest that the adrenergic response and insulin action are most likely blockaded by FAM132b mutants.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Bougneres P, Stunff CL, Pecqueur C, Pinglier E, Adnot P, Ricquier D. In vivo resistance of lipolysis to epinephrine. A new feature of childhood onset obesity. J Clin Investig. 1997;99:2568–73.
Qi Z, Ding S. Obesity-associated sympathetic overactivity in children and adolescents: the role of catecholamine resistance in lipid metabolism. J Pediatr Endocrinol Metab. 2016;29:113–25.
Seals DR, Bell C. Chronic sympathetic activation: consequence and cause of age-associated obesity? Diabetes. 2004;53:276–84.
Marion-Latard F, De Glisezinski I, Crampes F, Berlan M, Galitzky J, Suljkovicova H, et al. A single bout of exercise induces beta-adrenergic desensitization in human adipose tissue. Am J Physiol Regul Integr Comp Physiol. 2001;280:R166–R173.
Stich V, De Glisezinski I, Crampes F, Hejnova J, Cottet-Emard JM, Galitzky J, et al. Activation of alpha(2)-adrenergic receptors impairs exercise-induced lipolysis in SCAT of obese subjects. Am J Physiol Regul Integr Comp Physiol. 2000;279:R499–R504.
Perry RJ, Zhang XM, Zhang D, Kumashiro N, Camporez JP, Cline GW, et al. Leptin reverses diabetes by suppression of the hypothalamic-pituitary-adrenal axis. Nat Med. 2014;20:759–63.
Gan SU, Fu Z, Sia KC, Kon OL, Calne R, Lee KO. Development of a liver-specific Tet-off AAV8 vector for improved safety of insulin gene therapy for diabetes. J Gene Med. 2019;21:e3067.
Jimenez V, Jambrina C, Casana E, Sacristan V, Munoz S, Darriba S. et al. FGF21 gene therapy as treatment for obesity and insulin resistance. Embo Mol Med. 2018;8:e8791
Hoffmann JM, Grunberg JR, Church C, Elias I, Palsdottir V, Jansson JO, et al. BMP4 gene therapy in mature mice reduces BAT activation but protects from obesity by browning subcutaneous adipose tissue. Cell Rep. 2017;20:1038–49.
Samson SL, Gonzalez EV, Yechoor V, Bajaj M, Oka K, Chan L. Gene therapy for diabetes: metabolic effects of helper-dependent adenoviral exendin 4 expression in a diet-induced obesity mouse model. Mol Ther. 2008;16:1805–12.
Liang Q, Zhong L, Zhang J, Wang Y, Bornstein SR, Triggle CR, et al. FGF21 maintains glucose homeostasis by mediating the cross talk between liver and brain during prolonged fasting. Diabetes. 2014;63:4064–75.
Seldin MM, Peterson JM, Byerly MS, Wei Z, Wong GW. Myonectin (CTRP15), a novel myokine that links skeletal muscle to systemic lipid homeostasis. J Biol Chem. 2012;287:11968–80.
Mi Q, Li Y, Wang M, Yang G, Zhao X, Liu H, et al. Circulating C1q/TNF-related protein isoform 15 is a marker for the presence of metabolic syndrome. Diabetes Metab Res Rev. 2019;35:e3085.
Kautz L, Jung G, Du X, Gabayan V, Chapman J, Nasoff M, et al. Erythroferrone contributes to hepcidin suppression and iron overload in a mouse model of beta-thalassemia. Blood. 2015;126:2031–7.
Huang W, Queen NJ, McMurphy TB, Ali S, Cao L. Adipose PTEN regulates adult adipose tissue homeostasis and redistribution via a PTEN-leptin-sympathetic loop. Mol Metab. 2019;30:48–60.
Little HC, Rodriguez S, Lei X, Tan SY, Stewart AN, Sahagun A, et al. Myonectin deletion promotes adipose fat storage and reduces liver steatosis. FASEB J. 2019;33:8666–87.
Riaz M, Raz Y, Moloney EB, van Putten M, Krom YD, van der Maarel SM, et al. Differential myofiber-type transduction preference of adeno-associated virus serotypes 6 and 9. Skelet Muscle. 2015;5:37.
Seldin MM, Lei X, Tan SY, Stanson KP, Wei Z, Wong GW. Skeletal muscle-derived myonectin activates the mammalian target of rapamycin (mTOR) pathway to suppress autophagy in liver. J Biol Chem. 2013;288:36073–82.
Colella P, Ronzitti G, Mingozzi F. Emerging Issues in AAV-mediated in vivo gene therapy. Mol Ther Methods Clin Dev. 2018;8:87–104.
Seldin MM, Wong GW. Regulation of tissue crosstalk by skeletal muscle-derived myonectin and other myokines. Adipocyte. 2012;1:200–2.
Simcox J, Geoghegan G, Maschek JA, Bensard CL, Pasquali M, Miao R, et al. Global analysis of plasma lipids identifies liver-derived acylcarnitines as a fuel source for brown fat thermogenesis. Cell Metab. 2017;26:509–22.
Simcox JA, McClain DA. Iron and diabetes risk. Cell Metab. 2013;17:329–41.
Babitt JL. Erythroferrone in iron regulation and beyond. Blood. 2022;139:319–21.
Kautz L, Jung G, Valore EV, Rivella S, Nemeth E, Ganz T. Identification of erythroferrone as an erythroid regulator of iron metabolism. Nat Genet. 2014;46:678–84.
Schutten MT, Houben AJ, de Leeuw PW, Stehouwer CD. The link between adipose tissue renin-angiotensin-aldosterone system signaling and obesity-associated hypertension. Physiology. 2017;32:197–209.
Marcus Y, Shefer G, Stern N. Adipose tissue renin-angiotensin-aldosterone system (RAAS) and progression of insulin resistance. Mol Cell Endocrinol. 2013;378:1–14.
Liao WH, Suendermann C, Steuer AE, Pacheco LG, Odermatt A, Faresse N. et al. Aldosterone deficiency in mice burdens respiration and accentuates diet-induced hyperinsulinemia and obesity. JCI Insight. 2018;14:e99015
Nagareddy PR, Murphy AJ, Stirzaker RA, Hu Y, Yu S, Miller RG, et al. Hyperglycemia promotes myelopoiesis and impairs the resolution of atherosclerosis. Cell Metab. 2013;17:695–708.
Brownlee M. Hyperglycemia-stimulated myelopoiesis causes impaired regression of atherosclerosis in type 1 diabetes. Cell Metab. 2013;17:631–3.
Knudsen JG, Hamilton A, Ramracheya R, Tarasov AI, Brereton M, Haythorne E, et al. Dysregulation of glucagon secretion by hyperglycemia-induced sodium-dependent reduction of ATP production. Cell Metab. 2019;29:430–42.
Peters A, Schweiger U, Fruhwald-Schultes B, Born J, Fehm HL. The neuroendocrine control of glucose allocation. Exp Clin Endocrinol Diabetes. 2002;110:199–211.
Rojas JM, Matsen ME, Mundinger TO, Morton GJ, Stefanovski D, Bergman RN, et al. Glucose intolerance induced by blockade of central FGF receptors is linked to an acute stress response. Mol Metab. 2015;4:561–8.
Baskin KK, Winders BR, Olson EN. Muscle as a “mediator” of systemic metabolism. Cell Metab. 2015;21:237–48.
Zhao X, Karpac J. Muscle directs diurnal energy homeostasis through a myokine-dependent hormone module in drosophila. Curr Biol. 2017;27:1941–55.
Covington JD, Tam CS, Bajpeyi S, Galgani JE, Noland RC, Smith SR, et al. Myokine expression in muscle and myotubes in response to exercise stimulation. Med Sci Sports Exerc. 2016;48:384–90.
Jimenez V, Munoz S, Casana E, Mallol C, Elias I, Jambrina C, et al. In vivo adeno-associated viral vector-mediated genetic engineering of white and brown adipose tissue in adult mice. Diabetes. 2013;62:4012–22.
Cantley JL, Vatner DF, Galbo T, Madiraju A, Petersen M, Perry RJ, et al. Targeting steroid receptor coactivator 1 with antisense oligonucleotides increases insulin-stimulated skeletal muscle glucose uptake in chow-fed and high-fat-fed male rats. Am J Physiol Endocrinol Metab. 2014;307:E773–E783.
Gaidhu MP, Anthony NM, Patel P, Hawke TJ, Ceddia RB. Dysregulation of lipolysis and lipid metabolism in visceral and subcutaneous adipocytes by high-fat diet: role of ATGL, HSL, and AMPK. Am J Physiol Cell Physiol. 2010;298:C961–C971.
Qi Z, Xia J, Xue X, Liu J, Liu W, Ding S. Targeting viperin improves diet-induced glucose intolerance but not adipose tissue inflammation. Oncotarget. 2017;8:101418–36.
Roy A, Kucukural A, Zhang Y. I-TASSER: a unified platform for automated protein structure and function prediction. Nat Protoc. 2010;5:725–38.
Yang J, Yan R, Roy A, Xu D, Poisson J, Zhang Y. The I-TASSER Suite: protein structure and function prediction. Nat Methods. 2015;12:7–8.
Yang J, Zhang Y. I-TASSER server: new development for protein structure and function predictions. Nucleic Acids Res. 2015;43:W174–W181.
Laskowski RA, Rullmannn JA, MacArthur MW, Kaptein R, Thornton JM. AQUA and PROCHECK-NMR: programs for checking the quality of protein structures solved by NMR. J Biomol NMR. 1996;8:477–86.
Eargle J, Wright D, Luthey-Schulten Z. Multiple alignment of protein structures and sequences for VMD. Bioinformatics. 2006;22:504–6.
Acknowledgements
We thank Dr. Li Rui, the director engineer of Genomeditech (Shanghai) Co., Ltd., for designing and producing recombinant AAV vectors of serotype 9 encoding the FAM132b cDNA sequence or RNAi sequence. We thank Dr. Wu J.L. and Dr. Liu Y.M., technician of the Instrumental Analysis Center of Shanghai Jiaotong University, for their expertise and conversations related to body composition analyses and metabolic assays. Supported by Natural Science Foundation of China grants 31871208 and 31300977, Shanghai Natural Science Foundation (18ZR1412000), the Fundamental Research Funds for the Central Universities (40500-20104-222288) and the Key Laboratory Construction Project of Adolescent Health Assessment and Exercise Intervention of Ministry of Education, China (No. 40500-541235-14203/004).
Author information
Authors and Affiliations
Contributions
ZQ and WL conceived, designed, performed, and interpreted experiments and made figures. JX, XX, JL, WL, LL, ZC, and ZH performed animal care, glucose tolerance tests, insulin tolerance tests, EPI tolerance tests, real-time PCR, and data collection, interpreted a part of experiments and made some figures. XZ, QZ, XL, LC, BJ performed animal care, metabolite analysis, and data processing. YZ, JL performed structural analysis and ZDOCK. SD participated in interpreting the results and supervising the experimental plan. ZQ and WL wrote and revised the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Qi, Z., Xia, J., Xue, X. et al. Codon-optimized FAM132b gene therapy prevents dietary obesity by blockading adrenergic response and insulin action. Int J Obes 46, 1970–1982 (2022). https://doi.org/10.1038/s41366-022-01189-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41366-022-01189-x