Abstract
Background
Exercise is an important strategy in the management of diabetes. Experimental studies have shown that exercise acts, at least in part, by inducing the production of myokines that improve metabolic control and activate brown/beige adipose tissue depots. Combined training (CT) is recommended by the major diabetes guidelines due to its metabolic and cardiovascular benefits, however, its impact on brown/beige adipose tissue activities has never been tested in humans with overweight and type 2 diabetes (T2D). Here, we evaluated the effects of 16-week combined training (CT) program on brown adipose tissue activity; browning and autophagy markers, and serum pro-thermogenic/inflammatory inducers in patients with overweight and T2D.
Methods
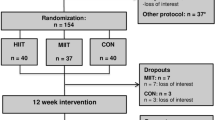
Thirty-four patients with overweight and T2D were assigned to either a control group (CG) or a combined training group (CTG) in a randomized and controlled study. Functional/fitness parameters, anthropometry/body composition parameters, blood hormone/biochemical parameters, thermogenic/autophagic gene expression in subcutaneous adipose tissue were evaluated before and at the end of the intervention. In addition, cold-induced 18-Fluoroxyglucose Positron Emission Computed Tomography (18F-FDG PET/CT) was performed in the training group before and after the end of the intervention.
Results
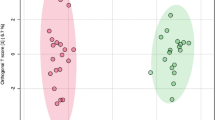
CT increased cervical/supraclavicular brown adipose tissue (BAT) thermogenic activity (p = 0.03) as well as in perirenal adipose tissue (p = 0.02). In addition, CT increased the expression of genes related to thermogenic profile (TMEM26: + 95%, p = 0.04; and EPSTI1: + 26%, p = 0.03) and decreased autophagic genes (ULK1: −15%, p = 0.04; LC3: −5%, p = 0.02; and ATG4: −22%, p < 0.001) in subcutaneous adipose tissue. There were positive correlations between Δ% BAT activity with Δ% of post training energy expenditure cold exposure, HDL-c, IL4, adiponectin, irisin, meteorin-like, and TMEM26 and ZIC1 genes, besides negative correlations with LDL-c, total cholesterol and C-reactive protein.
Conclusion
This is the first evidence of the beneficial actions of CT on adipose tissue thermogenic activity in humans, and it adds important support for the recommendation of CT as a strategy in the management of diabetes.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Cypess AM, Lehman S, Williams G, Tal I, Rodman D, Goldfine AB, et al. Identification and importance of brown adipose tissue in adult humans. N Engl J Med. 2009;360:1509–17.
Virtanen KA, Lidell ME, Orava J, Heglind M, Westergren R, Niemi T, et al. Functional brown adipose tissue in healthy adults. N Engl J Med. 2009;360:1518–25.
Villarroya F, Cereijo R, Gavalda-Navarro A, Villarroya J, Giralt M. Inflammation of brown/beige adipose tissues in obesity and metabolic disease. J Intern Med. 2018;284:492–504.
Chen KY, Brychta RJ, Abdul Sater Z, Cassimatis TM, Cero C, Fletcher LA, et al. Opportunities and challenges in the therapeutic activation of human energy expenditure and thermogenesis to manage obesity. J Biol Chem. 2020;295:1926–42.
Hanssen MJ, Hoeks J, Brans B, van der Lans AA, Schaart G, van den Driessche JJ, et al. Short-term cold acclimation improves insulin sensitivity in patients with type 2 diabetes mellitus. Nat Med. 2015;21:863–5.
Blondin DP, Labbe SM, Noll C, Kunach M, Phoenix S, Guerin B, et al. Selective Impairment of Glucose but Not Fatty Acid or Oxidative Metabolism in Brown Adipose Tissue of Subjects With Type 2 Diabetes. Diabetes. 2015;64:2388–97.
Kushner RF, Ryan DH. Assessment and lifestyle management of patients with obesity: clinical recommendations from systematic reviews. Jama. 2014;312:943–52.
Colberg SR, Albright AL, Blissmer BJ, Braun B, Chasan-Taber L, Fernhall B, et al. Exercise and type 2 diabetes: American College of Sports Medicine and the American Diabetes Association: joint position statement. Exercise and type 2 diabetes. Med Sci Sports Exercise. 2010;42:2282–303.
Colberg SR, Sigal RJ, Yardley JE, Riddell MC, Dunstan DW, Dempsey PC, et al. Physical Activity/Exercise and Diabetes: A Position Statement of the American Diabetes Association. Diabetes Care. 2016;39:2065–79.
Gleeson M, Bishop NC, Stensel DJ, Lindley MR, Mastana SS, Nimmo MA. The anti-inflammatory effects of exercise: mechanisms and implications for the prevention and treatment of disease. Nat Rev Immunol. 2011;11:607–15.
Sylow L, Kleinert M, Richter EA, Jensen TE. Exercise-stimulated glucose uptake - regulation and implications for glycaemic control. Nat Rev Endocrinol. 2017;13:133–48.
Enerback S. Brown adipose tissue in humans. Intern J Obesity. 2010;34:S43–6.
Stanford KI, Middelbeek RJ, Townsend KL, Lee MY, Takahashi H, So K, et al. A novel role for subcutaneous adipose tissue in exercise-induced improvements in glucose homeostasis. Diabetes. 2015;64:2002–14.
May FJ, Baer LA, Lehnig AC, So K, Chen EY, Gao F, et al. Lipidomic Adaptations in White and Brown Adipose Tissue in Response to Exercise Demonstrate Molecular Species-Specific Remodeling. Cell Rep. 2017;18:1558–72.
Bostrom P, Wu J, Jedrychowski MP, Korde A, Ye L, Lo JC, et al. A PGC1-alpha-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature. 2012;481:463–8.
Pedersen BK, Febbraio MA. Muscles, exercise and obesity: skeletal muscle as a secretory organ. Nat Rev Endocrinol. 2012;8:457–65.
Dewal RS, Stanford KI. Effects of exercise on brown and beige adipocytes. Biochimica et Biophysica Acta. Mol Cell Biol Lipids. 2019;1864:71–78.
Dinas PC, Nikaki A, Jamurtas AZ, Prassopoulos V, Efthymiadou R, Koutedakis Y, et al. Association between habitual physical activity and brown adipose tissue activity in individuals undergoing PET-CT scan. Clin Endocrinol. 2015;82:147–54.
Singhal V, Maffazioli GD, Ackerman KE, Lee H, Elia EF, Woolley R, et al. Effect of Chronic Athletic Activity on Brown Fat in Young Women. PloS ONE. 2016;11:e0156353.
Vosselman MJ, Hoeks J, Brans B, Pallubinsky H, Nascimento EB, van der Lans AA, et al. Low brown adipose tissue activity in endurance-trained compared with lean sedentary men. Intern J Obesity. 2015;39:1696–702.
Motiani P, Virtanen KA, Motiani KK, Eskelinen JJ, Middelbeek RJ, Goodyear LJ, et al. Decreased insulin-stimulated brown adipose tissue glucose uptake after short-term exercise training in healthy middle-aged men. Diabetes Obesity Metab. 2017;19:1379–88.
Norheim F, Langleite TM, Hjorth M, Holen T, Kielland A, Stadheim HK, et al. The effects of acute and chronic exercise on PGC-1alpha, irisin and browning of subcutaneous adipose tissue in humans. FEBS J. 2014;281:739–49.
Tsiloulis T, Carey AL, Bayliss J, Canny B, Meex RCR, Watt MJ. No evidence of white adipocyte browning after endurance exercise training in obese men. Intern J Obesity. 2018;42:721–7.
Nakhuda A, Josse AR, Gburcik V, Crossland H, Raymond F, Metairon S, et al. Biomarkers of browning of white adipose tissue and their regulation during exercise- and diet-induced weight loss. Am J Clin Nutr. 2016;104:557–65.
Pino MF, Parsons SA, Smith SR, Sparks LM. Active individuals have high mitochondrial content and oxidative markers in their abdominal subcutaneous adipose tissue. Obesity. 2016;24:2467–70.
American Diabetes A. Standards of Medical Care in Diabetes-2019 Abridged for Primary Care Providers. Clin Diabetes: Publ Am Diabetes Assoc. 2019;37:11–34.
Villareal DT, Aguirre L, Gurney AB, Waters DL, Sinacore DR, Colombo E, et al. Aerobic or Resistance Exercise, or Both, in Dieting Obese Older Adults. N Engl J Med. 2017;376:1943–55.
Libardi CA, De Souza GV, Cavaglieri CR, Madruga VA, Chacon-Mikahil MP. Effect of resistance, endurance, and concurrent training on TNF-alpha, IL-6, and CRP. Med Sci Sports Exercise. 2012;44:50–6.
Brunelli DT, Chacon-Mikahil MP, Gaspari AF, Lopes WA, Bonganha V, Bonfante IL, et al. Combined Training Reduces Subclinical Inflammation in Obese Middle-Age Men. Med Sci Sports Exercise. 2015;47:2207–15.
Bonfante IL, Chacon-Mikahil MP, Brunelli DT, Gaspari AF, Duft RG, Lopes WA, et al. Combined training, FNDC5/irisin levels and metabolic markers in obese men: a randomised controlled trial. Eur J Sport Sci. 2017;17:629–37.
Duft RG, Castro A, Bonfante ILP, Brunelli DT, Chacon-Mikahil MPT, Cavaglieri CR. Metabolomics Approach in the Investigation of Metabolic Changes in Obese Men after 24 Weeks of Combined Training. J Proteome Res. 2017;16:2151–9.
Duft RG, Castro A, Bonfante ILP, Lopes WA, da Silva LR, Chacon-Mikahil MPT, et al. Altered metabolomic profiling of overweight and obese adolescents after combined training is associated with reduced insulin resistance. Sci Rep. 2020;10:16880.
Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–9.
Chen KY, Cypess AM, Laughlin MR, Haft CR, Hu HH, Bredella MA, et al. Brown Adipose Reporting Criteria in Imaging STudies (BARCIST 1.0): Recommendations for Standardized FDG-PET/CT Experiments in Humans. Cell Metab. 2016;24:210–22.
de-Lima-Junior JC, Souza GF, Moura-Assis A, Gaspar RS, Gaspar JM, Rocha AL, et al. Abnormal brown adipose tissue mitochondrial structure and function in IL10 deficiency. EBioMedicine. 2019;39:436–47.
Lahesmaa M, Eriksson O, Gnad T, Oikonen V, Bucci M, Hirvonen J, et al. Cannabinoid Type 1 Receptors Are Upregulated During Acute Activation of Brown Adipose Tissue. Diabetes. 2018;67:1226–36.
UD M, Raiko J, Saari T, Saunavaara V, Kudomi N, Solin O, et al. Human Brown Fat Radiodensity Indicates Underlying Tissue Composition and Systemic Metabolic Health. J Clin Endocrinol Metab. 2017;102:2258–67.
Acosta FM, Martinez-Tellez B, Sanchez-Delgado G, Migueles JH, Contreras-Gomez MA, Martinez-Avila WD, et al. Association of Objectively Measured Physical Activity With Brown Adipose Tissue Volume and Activity in Young Adults. J Clin Endocrinol Metab. 2019;104:223–33.
Nookaew I, Svensson PA, Jacobson P, Jernas M, Taube M, Larsson I, et al. Adipose tissue resting energy expenditure and expression of genes involved in mitochondrial function are higher in women than in men. J Clin Endocrinol Metab. 2013;98:E370–8.
Pfannenberg C, Werner MK, Ripkens S, Stef I, Deckert A, Schmadl M, et al. Impact of age on the relationships of brown adipose tissue with sex and adiposity in humans. Diabetes. 2010;59:1789–93.
Barquissau V, Leger B, Beuzelin D, Martins F, Amri EZ, Pisani DF, et al. Caloric Restriction and Diet-Induced Weight Loss Do Not Induce Browning of Human Subcutaneous White Adipose Tissue in Women and Men with Obesity. Cell Rep. 2018;22:1079–89.
Lee MW, Odegaard JI, Mukundan L, Qiu Y, Molofsky AB, Nussbaum JC, et al. Activated type 2 innate lymphoid cells regulate beige fat biogenesis. Cell. 2015;160:74–87.
Qian SW, Tang Y, Li X, Liu Y, Zhang YY, Huang HY, et al. BMP4-mediated brown fat-like changes in white adipose tissue alter glucose and energy homeostasis. Proc Natl Acad Sci USA. 2013;110:E798–807.
Vijay J, Gauthier MF, Biswell RL, Louiselle DA, Johnston JJ, Cheung WA, et al. Single-cell analysis of human adipose tissue identifies depot and disease specific cell types. Nat Metab. 2020;2:97–109.
Hui X, Gu P, Zhang J, Nie T, Pan Y, Wu D, et al. Adiponectin Enhances Cold-Induced Browning of Subcutaneous Adipose Tissue via Promoting M2 Macrophage Proliferation. Cell Metab. 2015;22:279–90.
Song A, Dai W, Jang MJ, Medrano L, Li Z, Zhao H, et al. Low- and high-thermogenic brown adipocyte subpopulations coexist in murine adipose tissue. J Clin Investig. 2020;130:247–57.
Cohen P, Spiegelman BM. Brown and beige fat: molecular parts of a thermogenic machine. Diabetes. 2015;64:2346–51.
Jespersen NZ, Larsen TJ, Peijs L, Daugaard S, Homoe P, Loft A, et al. A classical brown adipose tissue mRNA signature partly overlaps with brite in the supraclavicular region of adult humans. Cell Metab. 2013;17:798–805.
Walden TB, Hansen IR, Timmons JA, Cannon B, Nedergaard J. Recruited vs. nonrecruited molecular signatures of brown, “brite,” and white adipose tissues. Am J Physiol Endocrinol Metab. 2012;302:E19–31.
Singh R, Xiang Y, Wang Y, Baikati K, Cuervo AM, Luu YK, et al. Autophagy regulates adipose mass and differentiation in mice. J Clin Investig. 2009;119:3329–39.
Parray HA, Yun JW. Combined inhibition of autophagy protein 5 and galectin-1 by thiodigalactoside reduces diet-induced obesity through induction of white fat browning. IUBMB Life. 2017;69:510–21.
Nunez CE, Rodrigues VS, Gomes FS, Moura RF, Victorio SC, Bombassaro B, et al. Defective regulation of adipose tissue autophagy in obesity. Int J Obesity. 2013;37:1473–80.
Wagner MJ, Kim TH, Kadmon J, Nguyen ND, Ganguli S, Schnitzer MJ, et al. Shared Cortex-Cerebellum Dynamics in the Execution and Learning of a Motor Task. Cell. 2019;177:669–82. e24
Achari NK, al-Ubaidy S, Downman CB. Cerebellar initiation of discharges in sympathetic nerves. J Physiol. 1971;215:21P–22P.
Paton JF, Spyer KM. Brain stem regions mediating the cardiovascular responses elicited from the posterior cerebellar cortex in the rabbit. J Physiol. 1990;427:533–52.
Rodriguez A, Catalan V, Ramirez B, Unamuno X, Portincasa P, Gomez-Ambrosi J, et al. Impact of adipokines and myokines on fat browning. J Physiol Biochem. 2020;76:227–40.
Acknowledgements
ILPB thank Luiz Carlos Bonfante (In memoriam). The authors thank the participants; Obesity and Comorbidities Research Center—University of Campinas; Clinics Hospital—University of Campinas; Faculty of Physical Education—University of Campinas; Nuclear Medicine and collaborators team of Clinics Hospital—University of Campinas; Integrated Teaching, Research and Extension Laboratory—School of Physical Education—University of Campinas; Bárbara J. Amorim, Sergio Brunetto, Edna Brunetto, Natália Tobar, Antonio Calixto and Marcela Reymond Simões for your contribution in technical support. Finally we thank the funding agencies, cited mentioned below.
Funding
São Paulo State Research Support Foundation (FAPESP)—São Paulo/Brazil—Regular Research Grants—Process: 2016/08751-3; Coordination for the Improvement of Higher Education Personnel (CAPES) - Brazil—Financing Code 001; National Council for Scientific and Technological Development (CNPQ) – Brazil - Financing Code 303571/2018-7.
Author information
Authors and Affiliations
Contributions
CRC supervised the study. ILPB, MMP, RGD, KCSM, JCLJ, JCST, EARF, DTB, JM, CDR, MPTCM, LAV, and CRC performed hypothesis, generation, contributed to the design, data analysis, interpretation of results and paper preparation. ILPB, MMP, RGD, KCSM, JCLJ, EARF, DTB, JABL, JM, MLB, TMFA conducted experiments, tests, training sessions, and data analysis. All authors edited and approved the final paper. ILPB is the guarantor of this work and, as such, had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Bonfante, I.L.P., Monfort-Pires, M., Duft, R.G. et al. Combined training increases thermogenic fat activity in patients with overweight and type 2 diabetes. Int J Obes 46, 1145–1154 (2022). https://doi.org/10.1038/s41366-022-01086-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41366-022-01086-3