Abstract
Objectives
Maternal overfeeding during gestation may lead to adverse metabolic programming in the offspring mediated by epigenetic alterations. Potential reversal, in early life, of these alterations may help in the prevention of future cardio-metabolic conditions. In this context, our aims were: (1) to study the effects of maternal overfeeding on the metabolic and epigenetic programming of offspring’s adipose tissue; and (2) to test the potential of postnatal metformin treatment to reverse these changes.
Methods
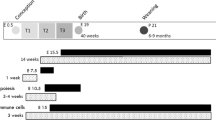
We used a swine animal model where commercial production sows were either overfed or kept under standard diet during gestation, and piglets at birth were randomly assigned to metformin (n = 16 per group) or vehicle treatment during lactation (n = 16 per group).
Results
Piglets born to overfed sows showed a worse metabolic profile (higher weight, weight gain from birth and abdominal circumference; all p < 0.05) together with altered serological markers (increased HOMA-IR, fructosamine, total cholesterol, C-Reactive Protein and lower HMW adiponectin; all p < 0.05). The visceral adipose tissue also showed altered morphology (increased adipocyte area, perimeter and diameter; all p < 0.05), as well as changes in gene expression (higher CCL2 and INSR, lower DLK1; all p < 0.05), and in DNA methylation (96 hypermethylated and 99 hypomethylated CpG sites; FDR < 0.05). Metformin treatment significantly ameliorated the abnormal metabolic profile, decreasing piglets’ weight, weight gain from birth, abdominal circumference and fructosamine (all p < 0.05) and reduced adipocyte area, perimeter, and diameter in visceral adipose tissue (all p < 0.05). In addition, metformin treatment potentiated several associations between gene expression in visceral adipose tissue and the altered metabolic markers.
Conclusions
Maternal overfeeding during gestation leads to metabolic abnormalities in the offspring, including adipose tissue alterations. Early metformin treatment mitigates these effects and could help rescue the offspring’s metabolic health.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Chen C, Xu X, Yan Y. Estimated global overweight and obesity burden in pregnant women based on panel data model. PLoS ONE. 2018;13:1–14.
Elshenawy S, Simmons R. Maternal obesity and prenatal programming. Mol Cell Endocrinol [Internet]. 2016;435:2–6. https://doi.org/10.1016/j.mce.2016.07.002.
Lahti-Pulkkinen M, Bhattacharya S, Wild SH, Lindsay RS, Räikkönen K, Norman JE, et al. Consequences of being overweight or obese during pregnancy on diabetes in the offspring: a record linkage study in Aberdeen, Scotland. Diabetologia. 2019;62:1412–9.
Mamun AA, O’Callaghan M, Callaway L, Williams G, Najman J, Lawlor DA. Associations of gestational weight gain with offspring body mass index and blood pressure at 21 years of ageevidence from a birth cohort study. Circulation. 2009;119:1720–7.
Gaillard R, Steegers EAP, Franco OH, Hofman A, Jaddoe VWV. Maternal weight gain in different periods of pregnancy and childhood cardio-metabolic outcomes. The Generation R Study. Int J Obes. 2015;39:677–85.
Godfrey KM, Reynolds RM, Prescott SL, Nyirenda M, Jaddoe VWV, Eriksson JG, et al. Influence of maternal obesity on the long-term health of offspring. Lancet Diabetes Endocrinol. 2017;5:53–64.
Ma X, Kang S. Functional implications of DNA methylation in adipose biology. Diabetes. 2019;68:871–8.
Zhong T, Men Y, Lu L, Geng T, Zhou J, Mitsuhashi A, et al. Metformin alters DNA methylation genome-wide via the H19/SAHH axis. Oncogene. 2017;36:2345–54.
Foretz M, Guigas B, Viollet B. Understanding the glucoregulatory mechanisms of metformin in type 2 diabetes mellitus. Nat Rev Endocrinol [Internet]. 2019;15:569–89. https://doi.org/10.1038/s41574-019-0242-2.
Khokhar A, Umpaichitra V, Chin VL, Perez-Colon S. Metformin use in children and adolescents with prediabetes. Pediatr Clin North Am [Internet]. 2017;64:1341–53. https://doi.org/10.1016/j.pcl.2017.08.010.
Koopmans SJ, Schuurman T. Considerations on pig models for appetite, metabolic syndrome and obese type 2 diabetes: From food intake to metabolic disease. Eur J Pharmacol [Internet]. 2015;759:231–9. https://doi.org/10.1016/j.ejphar.2015.03.044.
Astiz S, Gonzalez-Bulnes A, Astiz I, Barbero A, Perez-Solana ML, Garcia-Real I. Advanced onset of puberty after metformin therapy in swine with thrifty genotype. Exp Physiol. 2014;99:1241–52.
Eusebi PG, González-Prendes R, Quintanilla R, Tibau J, Cardoso TF, Clop A, et al. A genome-wide association analysis for carcass traits in a commercial Duroc pig population. Anim Genet. 2017;48:466–9.
Barbero A, Astiz S, Lopez-Bote CJ, Perez-Solana ML, Ayuso M, Garcia-Real I, et al. Maternal malnutrition and offspring sex determine juvenile obesity and metabolic disorders in a swine model of leptin resistance. PLoS ONE. 2013;8:1–14.
Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis [Internet]. Vol. 9, Nature Methods. Nature Publishing Group; 2012 [cited 2021 Sep 16]. p. 671–5. Available from: https://www.nature.com/articles/nmeth.2089.
ModENCODE E a. Guidelines For Experiments Generating ChIP, DNase, FAIRE, and DNA Methylation Genome Wide Location Data [Internet]. 2011. Available from: https://genome.ucsc.edu/ENCODE/experiment_guidelines.html.
Rosenbloom KR, Armstrong J, Barber GP, Casper J, Clawson H, Diekhans M, et al. The UCSC Genome Browser database: 2015 update. Nucleic Acids Res. 2015;43:D670–81.
Groenen MAM, Archibald AL, Uenishi H, Tuggle CK, Takeuchi Y, Rothschild MF, et al. Analyses of pig genomes provide insight into porcine demography and evolution. Nature. 2012;491:393–8.
Andrews, S. FastQC: A Quality Control Tool for High Throughput Sequence Data [Online]. 2010. Available online at: http://www.bioinformatics.babraham.ac.uk/projects/fastqc/.
Krueger F. Trim Galore! [http://www.bioinformatics.babraham.ac.uk/projects/trim_galore/].
Krueger F, Andrews SR. Bismark: a flexible aligner and methylation caller for Bisulfite-Seq applications. Bioinformatics. 2011;27:1571–2.
Akalin A, Kormaksson M, Li S, Garrett-Bakelman FE, Figueroa ME, Melnick A, et alhttp://genomebiology.com/2012/13/10/R87. MethylKit: a comprehensive R package for the analysis of genome-wide DNA methylation profiles. Genome Biol [Internet]. 2012;13:R87 .
Mamun AA, Mannan M, Doi SAR. Gestational weight gain in relation to offspring obesity over the life course: a systematic review and bias-adjusted meta-analysis. Obes Rev. 2014;15:338–47.
Fraser A, Tilling K, MacDonald-Wallis C, Sattar N, Brion M-J, Benfield L, et al. Association of maternal weight gain in pregnancy with offspring obesity and metabolic and vascular traits in childhood. Circulation [Internet]. 2010;121:2557–64. http://ovidsp.ovid.com/ovidweb.cgi?T=JS&PAGE=reference&D=emed12&NEWS=N&AN=50951677.
Mitanchez D, Chavatte-Palmer P. Review shows that maternal obesity induces serious adverse neonatal effects and is associated with childhood obesity in their offspring. Acta Paediatr Int J Paediatr. 2018;107:1156–65.
Oken E, Taveras EM, Kleinman KP, Rich-Edwards JW, Gillman MW. Gestational weight gain and child adiposity at age 3 years. Am J Obstet Gynecol. 2007;196:322.e1–8.
Hull HR, Thornton JC, Ji Y, Paley C, Rosenn B, Mathews P. .et al. Higher infant body fat with excessive gestational weight gain in overweight women. Am J Obstet Gynecol [Internet]. 2011 [cited. 2021 Sep 16];205:211.e1–7. Available from: https://pubmed.ncbi.nlm.nih.gov/21621185/.
Crozier SR, Inskip HM, Godfrey KM, Cooper C, Harvey NC, Cole ZA, et al. Weight gain in pregnancy and childhood body composition: Findings from the Southampton Women’s Survey. Am J Clin Nutr. 2010;91:1745–51.
Badon SE, Dyer AR, Josefson JL. Gestational weight gain and neonatal adiposity in the hyperglycemia and adverse pregnancy outcome study-North American region. Obesity [Internet]. 2014;22:1731–8. https://pubmed.ncbi.nlm.nih.gov/24634400/.
Gonzalez-Bulnes A, Astiz S, Ovilo C, Lopez-Bote CJ, Sanchez-Sanchez R, Perez-Solana ML, et al. Early-postnatal changes in adiposity and lipids profile by transgenerational developmental programming in swine with obesity/leptin resistance. J Endocrinol. 2014;223:M17–29.
Arentson-Lantz EJ, Buhman KK, Ajuwon K, Donkin SS. Excess pregnancy weight gain leads to early indications of metabolic syndrome in a swine model of fetal programming. Nutr Res [Internet]. 2014;34:241–9. https://doi.org/10.1016/j.nutres.2014.01.001.
Rogozińska E, Marlin N, Jackson L, Rayanagoudar G, Ruifrok AE, Dodds J, et al. Effects of antenatal diet and physical activity on maternal and fetal outcomes: Individual patient data meta-analysis and health economic evaluation. Health Technol Assess (Rockv). 2017;21.
Nathanielsz PW, Ford SP, Long NM, Vega CC, Reyes-Castro LA, Zambrano E. Interventions to prevent adverse fetal programming due to maternal obesity during pregnancy. Nutr Rev. 2013;71:1–19.
Crozier SR, Robinson SM, Godfrey KM, Cooper C, Inskip HM. Women’s dietary patterns change little from before to during pregnancy. J Nutr. 2009;139:1956–63.
Catalano P, Demouzon SH. Maternal obesity and metabolic risk to the offspring: why lifestyle interventions may have not achieved the desired outcomes. Int J Obes (Lond). 2015;39:642–9.
Rowan JA, Hague WM, Gao W, Battin MR, Moore MP. Metformin versus insulin for the treatment of gestational diabetes. Obstet Gynecol Surv. 2008;63:616–8.
Romero R, Erez O, Hüttemann M, Maymon E, Panaitescu B, Conde-Agudelo A, et al. Metformin, the aspirin of the 21st century: its role in gestational diabetes mellitus, prevention of preeclampsia and cancer, and the promotion of longevity. Am J Obstet Gynecol. 2017;217:282–302.
Barbour LA, Scifres C, Valent AM, Friedman JE, Buchanan TA, Coustan D, et al. A cautionary response to SMFM statement: pharmacological treatment of gestational diabetes. Am J Obstet Gynecol. 2018;219:367 e361–7.
Priya G, Kalra S. Metformin in the management of diabetes during pregnancy and lactation. Drugs Context. 2018;7:1–21.
Bridgeman SC, Ellison GC, Melton PE, Newsholme P, Mamotte CDS. Epigenetic effects of metformin: From molecular mechanisms to clinical implications. Diabetes Obes Metab. 2018;20:1553–62.
Briggs GG, Ambrose PJ, Nageotte MP, Padilla G, Wan S. Excretion of metformin into breast milk and the effect on nursing infants. Obstet Gynecol. 2005;105:1437–41.
Agosti M, Tandoi F, Morlacchi L, Bossi A. Nutritional and metabolic programming during the first thousand days of life. Pediatr Med Chir. 2017;39:157.
Yerevanian A, Soukas AA. Metformin: mechanisms in human obesity and weight loss. Curr Obes Rep. 2019;8:156–64.
Shrestha D, Ouidir M, Wirkalemahu T, Zeng X, Tekola-Ayele F. Placental DNA methylation changes associated with maternal pre-pregnancy BMI and gestational weight gain. Int J Obes. 2020;44:1406–16.
Bohlin J, Andreassen BK, Joubert BR, Magnus MC, Wu MC, Parr CL, et al. Effect of maternal gestational weight gain on offspring DNA methylation: a follow-up to the ALSPAC cohort study. BMC Res Notes. 2015;8:1–5.
Morales E, Groom A, Lawlor DA, Relton CL. DNA methylation signatures in cord blood associated with maternal gestational weight gain: results from the ALSPAC cohort. BMC Res Notes. 2014;7:1–10.
Borengasser SJ, Zhong Y, Kang P, Lindsey F, Ronis MJJ, Badger TM, et al. Maternal obesity enhances white adipose tissue differentiation and alters genome-scale DNA methylation in male rat offspring. Endocrinology. 2013;154:4113–25.
Alsayegh KN, Sheridan SD, Iyer S, Rao RR. Knockdown of CDK2AP1 in human embryonic stem cells reduces the threshold of differentiation. PLoS ONE. 2018;13:1–16.
Márquez-Quiñones A, Mutch DM, Debard C, Wang P, Combes M, Roussel B, et al. Adipose tissue transcriptome reflects variations between subjects with continued weight loss and subjects regaining weight 6 mo after caloric restriction independent of energy intake. Am J Clin Nutr. 2010;92:975–84.
Grempler R, Augustin R, Froehner S, Hildebrandt T, Simon E, Mark M, et al. Functional characterisation of human SGLT-5 as a novel kidney-specific sodium-dependent sugar transporter. FEBS Lett. 2012;586:248–53.
Carobbio S, Hagen RM, Lelliott CJ, Slawik M, Medina-Gomez G, Tan CY, et al. Adaptive changes of the Insig1/SREBP1/SCD1 set point help adipose tissue to cope with increased storage demands of obesity. Diabetes. 2013;62:3697–708.
Pinnick KE, Karpe F. DNA methylation of genes in adipose tissue. Proc Nutr Soc. 2011;70:57–63.
Kim M. DNA methylation: a cause and consequence of type 2 diabetes. Genomics Inform. 2019;17:e38.
Wahl S, Drong A, Lehne B, Loh M, Scott WR, Kunze S, et al. Epigenome-wide association study of body mass index, and the adverse outcomes of adiposity. Nature. 2017;541:81–6.
Acknowledgements
SX-T holds a Sara Borrell contract from Carlos III National Institute of Health (ISCIII; CD15–00162). BM-P holds a contract from Generalitat de Catalunya (SLT002/16/00065). GC-B holds a Sara Borrell contract from Carlos III National Institute of Health (ISCIII; CD19-00172). JB is Miguel Servet investigator (ISCIII; CPII17/00013). LI is a Clinical Investigator of CIBERDEM (Spanish Biomedical Research Centre in Diabetes and Associated Metabolic Disorders), from ISCIII. AL-B is an I3 investigator (Spanish Ministry of Economy and Competitiveness). This study was supported by grants from the Ministerio de Ciencia e Innovación, Instituto de Salud Carlos III (ISCIII), Madrid, Spain (PI17/00557 to JB, and PI16/01335 and PI19/00451to AL-B), projects co-funded by FEDER (Fondo Europeo de Desarrollo Regional).
Author information
Authors and Affiliations
Contributions
SX-T designed research study, conducted experiments, analyzed the data and wrote the first draft of the manuscript. BM-P conducted experiments, analyzed the data and wrote the first draft of the manuscript. GC-B, EL-M, JT, JR, EP-G, AP-P conducted experiments, acquired data and reviewed the manuscript, FDZ, LI reviewed the manuscript. JB, AL-B designed research study and reviewed the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Xargay-Torrent, S., Mas-Parés, B., Carreras-Badosa, G. et al. Metabolic programming in the offspring after gestational overfeeding in the mother: toward neonatal rescuing with metformin in a swine model. Int J Obes 46, 1018–1026 (2022). https://doi.org/10.1038/s41366-022-01076-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41366-022-01076-5