Abstract
Objectives
Metabolic inflammation is a hallmark of obesity and related disorders, afflicting substantial morbidity and mortality to individuals worldwide. White visceral and subcutaneous adipose tissue not only serves as energy storage but also controls metabolism. Adipose tissue inflammation, commonly observed in human obesity, is considered a critical driver of metabolic perturbation while molecular hubs are poorly explored. Metabolic stress evoked by e.g. long-chain fatty acids leads to oxidative perturbation of adipocytes and production of inflammatory cytokines, fuelling macrophage infiltration and systemic low-grade inflammation. Glutathione peroxidase 4 (GPX4) protects against lipid peroxidation, accumulation of oxygen-specific epitopes and cell death, collectively referred to as ferroptosis. Here, we explore the function of adipocyte GPX4 in mammalian metabolism.
Methods
We studied the regulation and function of GPX4 in differentiated mouse adipocytes derived from 3T3-L1 fibroblasts. We generated two conditional adipocyte-specific Gpx4 knockout mice by crossing Gpx4fl/fl mice with Adipoq-Cre+ (Gpx4−/−AT) or Fabp4-Cre+ (Gpx4+/−Fabp4) mice. Both models were metabolically characterized by a glucose tolerance test and insulin resistance test, and adipose tissue lipid peroxidation, inflammation and cell death were assessed by quantifying oxygen-specific epitopes, transcriptional analysis of chemokines, quantification of F4/80+ macrophages and TUNEL labelling.
Results
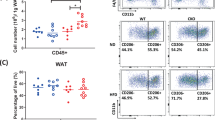
GPX4 expression was induced during and required for adipocyte differentiation. In mature adipocytes, impaired GPX4 activity spontaneously evoked lipid peroxidation and expression of inflammatory cytokines such as TNF-α, interleukin 1β (IL-1β), IL-6 and the IL-8 homologue CXCL1. Gpx4−/−AT mice spontaneously displayed adipocyte hypertrophy on a chow diet, which was paralleled by the accumulation of oxygen-specific epitopes and macrophage infiltration in adipose tissue. Furthermore, Gpx4−/−AT mice spontaneously developed glucose intolerance, hepatic insulin resistance and systemic low-grade inflammation, when compared to wildtype littermates, which was similarly recapitulated in Gpx4+/−Fabp4 mice. Gpx4−/−AT mice exhibited no signs of adipocyte death.
Conclusion
Adipocyte GPX4 protects against spontaneous metabolic dysregulation and systemic low-grade inflammation independent from ferroptosis, which could be therapeutically exploited in the future.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Heymsfield SB, Wadden TA. Mechanisms, pathophysiology, and management of obesity. N Engl J Med. 2017;376:254–66.
Dale CE, Fatemifar G, Palmer TM, White J, Prieto-Merino D, Zabaneh D, et al. Causal associations of adiposity and body fat distribution with coronary heart disease, stroke subtypes, and type 2 diabetes mellitus: a mendelian randomization analysis. Circulation. 2017;135:2373–88.
Bray GA, Kim KK, Wilding JPH. Obesity: a chronic relapsing progressive disease process. A position statement of the World Obesity Federation. Obes Rev. 2017;18:715–23.
Maier S, Wieland A, Cree-Green M, Nadeau K, Sullivan S, Lanaspa MA, et al. Lean NAFLD: an underrecognized and challenging disorder in medicine. Rev Endocr Metab Disord. 2021;22:351–66. https://doi.org/10.1007/s11154-020-09621-1.
Hotamisligil GS. Inflammation, metaflammation and immunometabolic disorders. Nature. 2017;542:177–85.
Tilg H, Zmora N, Adolph TE, Elinav E. The intestinal microbiota fuelling metabolic inflammation. Nat Rev Immunol. 2020;20:40–54.
Zwick RK, Guerrero-Juarez CF, Horsley V, Plikus MV. Anatomical, physiological, and functional diversity of adipose tissue. Cell Metab. 2018;27:68–83.
Friedman JM. Leptin and the endocrine control of energy balance. Nat Metab. 2019;1:754–64.
Straub LG, Scherer PE. Metabolic messengers: adiponectin. Nat Metab. 2019;1:334–9.
Saito M, Okamatsu-Ogura Y, Matsushita M, Watanabe K, Yoneshiro T, Nio-Kobayashi J, et al. High incidence of metabolically active brown adipose tissue in healthy adult humans: effects of cold exposure and adiposity. Diabetes. 2009;58:1526–31.
Reilly SM, Saltiel AR. Adapting to obesity with adipose tissue inflammation. Nat Rev Endocrinol. 2017;13:633–43.
Kotas ME, Medzhitov R. Homeostasis, inflammation, and disease susceptibility. Cell. 2015;160:816–27.
Lumeng CN, Saltiel AR. Inflammatory links between obesity and metabolic disease. J Clin Investig. 2011;121:2111–7.
Wen H, Gris D, Lei Y, Jha S, Zhang L, Huang MT, et al. Fatty acid-induced NLRP3-ASC inflammasome activation interferes with insulin signaling. Nat Immunol. 2011;12:408–15.
Saberi M, Woods NB, de Luca C, Schenk S, Lu JC, Bandyopadhyay G, et al. Hematopoietic cell-specific deletion of toll-like receptor 4 ameliorates hepatic and adipose tissue insulin resistance in high-fat-fed mice. Cell Metab. 2009;10:419–29.
Hotamisligil GS, Shargill NS, Spiegelman BM. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science. 1993;259:87–91.
Xu H, Barnes GT, Yang Q, Tan G, Yang D, Chou CJ, et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Investig. 2003;112:1821–30.
Nguyen MT, Favelyukis S, Nguyen AK, Reichart D, Scott PA, Jenn A, et al. A subpopulation of macrophages infiltrates hypertrophic adipose tissue and is activated by free fatty acids via Toll-like receptors 2 and 4 and JNK-dependent pathways. J Biol Chem. 2007;282:35279–92.
Houstis N, Rosen ED, Lander ES. Reactive oxygen species have a causal role in multiple forms of insulin resistance. Nature. 2006;440:944–8.
Ford ES, Mokdad AH, Giles WH, Brown DW. The metabolic syndrome and antioxidant concentrations: findings from the Third National Health and Nutrition Examination Survey. Diabetes. 2003;52:2346–52.
Doll S, Proneth B, Tyurina YY, Panzilius E, Kobayashi S, Ingold I, et al. ACSL4 dictates ferroptosis sensitivity by shaping cellular lipid composition. Nat Chem Biol. 2017;13:91–8.
Kagan VE, Mao G, Qu F, Angeli JP, Doll S, Croix CS, et al. Oxidized arachidonic and adrenic PEs navigate cells to ferroptosis. Nat Chem Biol. 2017;13:81–90.
Stockwell BR, Friedmann Angeli JP, Bayir H, Bush AI, Conrad M, Dixon SJ, et al. Ferroptosis: a regulated cell death nexus linking metabolism, redox biology, and disease. Cell. 2017;171:273–85.
Dixon SJ, Lemberg KM, Lamprecht MR, Skouta R, Zaitsev EM, Gleason CE, et al. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell. 2012;149:1060–72.
Viswanathan VS, Ryan MJ, Dhruv HD, Gill S, Eichhoff OM, Seashore-Ludlow B, et al. Dependency of a therapy-resistant state of cancer cells on a lipid peroxidase pathway. Nature. 2017;547:453–7.
Hangauer MJ, Viswanathan VS, Ryan MJ, Bole D, Eaton JK, Matov A, et al. Drug-tolerant persister cancer cells are vulnerable to GPX4 inhibition. Nature. 2017;551:247–50.
Friedmann Angeli JP, Schneider M, Proneth B, Tyurina YY, Tyurin VA, Hammond VJ, et al. Inactivation of the ferroptosis regulator Gpx4 triggers acute renal failure in mice. Nature Cell Biol. 2014;16:1180–91.
Mayr L, Grabherr F, Schwarzler J, Reitmeier I, Sommer F, Gehmacher T, et al. Dietary lipids fuel GPX4-restricted enteritis resembling Crohn’s disease. Nat Commun. 2020;11:1775.
Skouta R, Dixon SJ, Wang J, Dunn DE, Orman M, Shimada K, et al. Ferrostatins inhibit oxidative lipid damage and cell death in diverse disease models. J Am Chem Soc. 2014;136:4551–6.
Chen L, Hambright WS, Na R, Ran Q. Ablation of the ferroptosis inhibitor glutathione peroxidase 4 in neurons results in rapid motor neuron degeneration and paralysis. J Biol Chem. 2015;290:28097–106.
Sengupta A, Lichti UF, Carlson BA, Cataisson C, Ryscavage AO, Mikulec C, et al. Targeted disruption of glutathione peroxidase 4 in mouse skin epithelial cells impairs postnatal hair follicle morphogenesis that is partially rescued through inhibition of COX-2. J Investig Dermatol. 2013;133:1731–41.
Hambright WS, Fonseca RS, Chen L, Na R, Ran Q. Ablation of ferroptosis regulator glutathione peroxidase 4 in forebrain neurons promotes cognitive impairment and neurodegeneration. Redox Biol. 2017;12:8–17.
Katunga LA, Gudimella P, Efird JT, Abernathy S, Mattox TA, Beatty C, et al. Obesity in a model of gpx4 haploinsufficiency uncovers a causal role for lipid-derived aldehydes in human metabolic disease and cardiomyopathy. Mol Metab. 2015;4:493–506.
Long EK, Olson DM, Bernlohr DA. High-fat diet induces changes in adipose tissue trans-4-oxo-2-nonenal and trans-4-hydroxy-2-nonenal levels in a depot-specific manner. Free Radical Biology Med. 2013;63:390–8.
Kobayashi H, Matsuda M, Fukuhara A, Komuro R, Shimomura I. Dysregulated glutathione metabolism links to impaired insulin action in adipocytes. Am J Physiol Endocrinol Metab. 2009;296:E1326–34.
Rupérez AI, Olza J, Gil-Campos M, Leis R, Mesa MD, Tojo R, et al. Association of genetic polymorphisms for glutathione peroxidase genes with obesity in Spanish children. J Nutrigenet Nutrigenomics. 2014;7:130–42.
Villette S, Kyle JA, Brown KM, Pickard K, Milne JS, Nicol F, et al. A novel single nucleotide polymorphism in the 3’ untranslated region of human glutathione peroxidase 4 influences lipoxygenase metabolism. Blood Cells Mol Dis. 2002;29:174–8.
Yoo SE, Chen L, Na R, Liu Y, Rios C, Van Remmen H, et al. Gpx4 ablation in adult mice results in a lethal phenotype accompanied by neuronal loss in brain. Free Radical Biol Med. 2012;52:1820–7.
Eguchi J, Wang X, Yu S, Kershaw EE, Chiu PC, Dushay J, et al. Transcriptional control of adipose lipid handling by IRF4. Cell Metab. 2011;13:249–59.
He W, Barak Y, Hevener A, Olson P, Liao D, Le J, et al. Adipose-specific peroxisome proliferator-activated receptor gamma knockout causes insulin resistance in fat and liver but not in muscle. Proc Natl Acad Sci USA. 2003;100:15712–7.
Andrikopoulos S, Blair AR, Deluca N, Fam BC, Proietto J. Evaluating the glucose tolerance test in mice. Am J Physiol Endocrinol Metab. 2008;295:E1323–32.
Demetz E, Tancevski I, Duwensee K, Stanzl U, Huber E, Heim C, et al. Inhibition of hepatic scavenger receptor-class B type I by RNA interference decreases atherosclerosis in rabbits. Atherosclerosis. 2012;222:360–6.
Zebisch K, Voigt V, Wabitsch M, Brandsch M. Protocol for effective differentiation of 3T3-L1 cells to adipocytes. Anal Biochem. 2012;425:88–90.
Deshmane SL, Kremlev S, Amini S, Sawaya BE. Monocyte chemoattractant protein-1 (MCP-1): an overview. J Interferon Cytokine Res. 2009;29:313–26.
Blüher M. Obesity: global epidemiology and pathogenesis. Nat Rev Endocrinol. 2019;15:288–98.
Imai H, Hirao F, Sakamoto T, Sekine K, Mizukura Y, Saito M, et al. Early embryonic lethality caused by targeted disruption of the mouse PHGPx gene. Biochem Biophys Res Commun. 2003;305:278–86.
Carlson BA, Tobe R, Yefremova E, Tsuji PA, Hoffmann VJ, Schweizer U, et al. Glutathione peroxidase 4 and vitamin E cooperatively prevent hepatocellular degeneration. Redox Biology. 2016;9:22–31.
Stenkula KG, Erlanson-Albertsson C. Adipose cell size: importance in health and disease. Am J Physiol Regul Integr Comp Physiol. 2018;315:R284–r95.
Jo J, Gavrilova O, Pack S, Jou W, Mullen S, Sumner AE, et al. Hypertrophy and/or hyperplasia: dynamics of adipose tissue growth. PLoS Comput Biol. 2009;5:e1000324.
Longo M, Zatterale F, Naderi J, Parrillo L, Formisano P, Raciti GA, et al. Adipose tissue dysfunction as determinant of obesity-associated metabolic complications. Int J Mol Sci. 2019;20:2358. https://doi.org/10.3390/ijms20092358.
Wortmann M, Schneider M, Pircher J, Hellfritsch J, Aichler M, Vegi N, et al. Combined deficiency in glutathione peroxidase 4 and vitamin E causes multiorgan thrombus formation and early death in mice. Circulat Res. 2013;113:408–17.
Furukawa S, Fujita T, Shimabukuro M, Iwaki M, Yamada Y, Nakajima Y, et al. Increased oxidative stress in obesity and its impact on metabolic syndrome. J Clin Investig. 2004;114:1752–61.
Tatsumi F, Kaneto H, Hashiramoto M, Tawaramoto K, Obata A, Kimura T, et al. Anti-hypertensive azelnidipine preserves insulin signaling and glucose uptake against oxidative stress in 3T3-L1 adipocytes. Endocr J. 2015;62:741–7.
Pillon NJ, Croze ML, Vella RE, Soulère L, Lagarde M, Soulage CO. The lipid peroxidation by-product 4-hydroxy-2-nonenal (4-HNE) induces insulin resistance in skeletal muscle through both carbonyl and oxidative stress. Endocrinology. 2012;153:2099–111.
Merry TL, Tran M, Stathopoulos M, Wiede F, Fam BC, Dodd GT, et al. High-fat-fed obese glutathione peroxidase 1-deficient mice exhibit defective insulin secretion but protection from hepatic steatosis and liver damage. Antioxidants Redox Signal. 2014;20:2114–29.
Kendig EL, Chen Y, Krishan M, Johansson E, Schneider SN, Genter MB, et al. Lipid metabolism and body composition in Gclm(−/−) mice. Toxicol Appl Pharmacol. 2011;257:338–48.
Binder CJ, Papac-Milicevic N, Witztum JL. Innate sensing of oxidation-specific epitopes in health and disease. Nat Rev Immunol. 2016;16:485–97.
Bost F, Aouadi M, Caron L, Even P, Belmonte N, Prot M, et al. The extracellular signal-regulated kinase isoform ERK1 is specifically required for in vitro and in vivo adipogenesis. Diabetes. 2005;54:402–11.
Priest C, Tontonoz P. Inter-organ cross-talk in metabolic syndrome. Nat Metabol. 2019;1:1177–88.
Takahashi H, Alves CRR, Stanford KI, Middelbeek RJW, Pasquale N, Ryan RE, et al. TGF-β2 is an exercise-induced adipokine that regulates glucose and fatty acid metabolism. Nat Metab. 2019;1:291–303.
Ridker PM, Everett BM, Thuren T, MacFadyen JG, Chang WH, Ballantyne C, et al.Antiinflammatory therapy with canakinumab for atherosclerotic disease.N Engl J Med. 2017;377:1119–31.
Larsen CM, Faulenbach M, Vaag A, Vølund A, Ehses JA, Seifert B, et al. Interleukin-1-receptor antagonist in type 2 diabetes mellitus. N Engl J Med. 2007;356:1517–26.
Panahi Y, Hosseini MS, Khalili N, Naimi E, Majeed M, Sahebkar A. Antioxidant and anti-inflammatory effects of curcuminoid-piperine combination in subjects with metabolic syndrome: A randomized controlled trial and an updated meta-analysis. Clin Nutr. 2015;34:1101–8.
Acknowledgements
We are grateful for the support received from the Austrian Science Fund (FWF P33070) (to TEA). We appreciate the support of the Christian Doppler Research Foundation and the Austrian Federal Ministry of Science, Research and Economy and the National Foundation for Research, Technology and Development (to SK). We thank for the financial support of the Excellence Initiative (Competence Centres for Excellent Technologies—COMET) of the Austrian Research Promotion Agency FFG: Research Centre of Excellence in Vascular Ageing Tyrol, VASCage (K-Project Nr. 843536) funded by BMVIT, BMWFW, Wirtschaftsagentur Wien and Standortagentur Tirol (to HT). We are grateful for the support from the Austrian Society of Gastroenterology and Hepatology (ÖGGH) (to LM). We thank for the support from the Tyrolean Science Fund (TWF) (to FG).
Author information
Authors and Affiliations
Contributions
JS and LM designed, performed and analysed most experiments and helped prepare the manuscript together with and BR, FG, MP, BT, CG, BE and KS. AR and MH performed cholesterol quantification. QR and LAH provided an essential mouse model. HT, SK and TEA coordinated the project and prepared the manuscript together with JS and LM.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Schwärzler, J., Mayr, L., Radlinger, B. et al. Adipocyte GPX4 protects against inflammation, hepatic insulin resistance and metabolic dysregulation. Int J Obes 46, 951–959 (2022). https://doi.org/10.1038/s41366-022-01064-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41366-022-01064-9
This article is cited by
-
Molecular mechanisms of ferroptosis in cardiovascular disease
Molecular and Cellular Biochemistry (2024)
-
Inhibition of autophagy with chloroquine dysregulates mitochondrial quality control and energetics in adipocytes
Archives of Pharmacal Research (2022)