Abstract
Background
Pannexin 3 (PANX3) is a channel-forming glycoprotein that enables nutrient-induced inflammation in vitro, and genetic linkage data suggest that it regulates body mass index. Here, we characterized inflammatory and metabolic parameters in global Panx3 knockout (KO) mice in the context of forced treadmill running (FEX) and high-fat diet (HFD).
Methods
C57BL/6N (WT) and KO mice were randomized to either a FEX running protocol or no running (SED) from 24 until 30 weeks of age. Body weight was measured biweekly, and body composition was measured at 24 and 30 weeks of age. Male WT and KO mice were fed a HFD from 12 to 28 weeks of age. Metabolic organs were analyzed for a panel of inflammatory markers and PANX3 expression.
Results
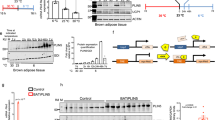
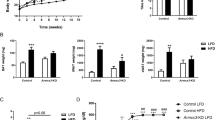
In females there were no significant differences in body composition between genotypes, which could be due to the lack of PANX3 expression in female white adipose tissue, while male KOs fed a chow diet had lower body weight and lower fat mass at 24 and 30 weeks of age, which was reduced to the same extent as 6 weeks of FEX in WT mice. In addition, male KO mice exhibited significantly lower expression of multiple pro-inflammatory genes in white adipose tissue compared to WT mice. While on a HFD body weight differences were insignificant, multiple inflammatory genes were significantly different in quadriceps muscle and white adipose tissue resulting in a more anti-inflammatory phenotype in KO mice compared to WT. The lower fat mass in male KO mice may be due to significantly fewer adipocytes in their subcutaneous fat compared to WT mice. Mechanistically, adipose stromal cells (ASCs) cultured from KO mice grow significantly slower than WT ASCs.
Conclusion
PANX3 is expressed in male adult mouse adipose tissue and may regulate adipocyte numbers, influencing fat accumulation and inflammation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
All data generated or analyzed during this study are included in the published article (and its online supplementary files). Raw data are available from the corresponding author upon reasonable request.
References
Romieu I, Dossus L, Barquera S, Blottière HM, Franks PW, Gunter M, et al. Energy balance and obesity: what are the main drivers? Cancer Causes Control. 2017;28:247–58.
Hall KD, Kahan S. Maintenance of lost weight and long-term management of obesity. Med Clin North Am. 2018;102:183–97.
Thaker VV. Genetic and epigenetics causes of obesity. Adolesc Med State Art Rev. 2017;28:379–405.
Penuela S, Gehi R, Laird DW. The biochemistry and function of pannexin channels. Biochim Biophys Acta. 2013;1828:15–22.
Tozzi M, Hansen JB, Novak I. Pannexin-1 mediated ATP release in adipocytes is sensitive to glucose and insulin and modulates lipolysis and macrophage migration. Acta Physiol (Oxf). 2020;228:e13360.
Lee VR, Barr KJ, Kelly JJ, Johnston D, Brown CFC, Robb KP, et al. Pannexin 1 regulates adipose stromal cell differentiation and fat accumulation. Sci Rep. 2018;8:16166.
Adamson SE, Meher AK, Chiu YH, Sandilos JK, Oberholtzer NP, Walker NN, et al. Pannexin 1 is required for full activation of insulin-stimulated glucose uptake in adipocytes. Mol Metab. 2015;4:610–8.
Senthivinayagam S, Serbulea V, Upchurch CM, Polanowska-Grabowska R, Mendu SK, Sahu S, et al. Adaptive thermogenesis in brown adipose tissue involves activation of pannexin-1 channels. Mol Metab. 2020;44:101130.
Halliwill KD, Quigley DA, Kang HC, Del Rosario R, Ginzinger D, Balmain A. Panx3 links body mass index and tumorigenesis in a genetically heterogeneous mouse model of carcinogen-induced cancer. Genome Med. 2016;8:83.
Sjölund J, Pelorosso FG, Quigley DA, DelRosario R, Balmain A. Identification of Hipk2 as an essential regulator of white fat development. Proc Natl Acad Sci USA. 2014;111:7373–8.
Pillon NJ, Li YE, Fink LN, Brozinick JT, Nikolayev A, Kuo MS, et al. Nucleotides released from palmitate-challenged muscle cells through pannexin-3 attract monocytes. Diabetes. 2014;63:3815–26.
Goossens GH. The metabolic phenotype in obesity: fat mass, body fat distribution, and adipose tissue function. Obes Facts. 2017;10:207–15.
Reilly SM, Saltiel AR. Adapting to obesity with adipose tissue inflammation. Nat Rev Endocrinol. 2017;13:633–43.
Stolarczyk E. Adipose tissue inflammation in obesity: a metabolic or immune response? Curr Opin Pharmacol. 2017;37:35–40.
Unamuno X, Gómez-Ambrosi J, Rodríguez A, Becerril S, Frühbeck G, Catalán V. Adipokine dysregulation and adipose tissue inflammation in human obesity. Eur J Clin Invest. 2018;48:e12997.
Burhans MS, Hagman DK, Kuzma JN, Schmidt KA, Kratz M. Contribution of adipose tissue inflammation to the development of type 2 diabetes mellitus. Compr Physiol. 2018;9:1–58.
Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006;444:860–7.
Kusminski CM, Bickel PE, Scherer PE. Targeting adipose tissue in the treatment of obesity-associated diabetes. Nat Rev Drug Discov. 2016;15:639–60.
Saxton SN, Clark BJ, Withers SB, Eringa EC, Heagerty AM. Mechanistic links between obesity, diabetes, and blood pressure: role of perivascular adipose tissue. Physiol Rev. 2019;99:1701–63.
Moon PM, Penuela S, Barr K, Khan S, Pin CL, Welch I, et al. Deletion of Panx3 prevents the development of surgically induced osteoarthritis. J Mol Med (Berl). 2015;93:845–56.
Abitbol JM, Kelly JJ, Barr K, Schormans AL, Laird DW, Allman BL. Differential effects of pannexins on noise-induced hearing loss. Biochem J. 2016;473:4665–80.
Penuela S, Bhalla R, Gong XQ, Cowan KN, Celetti SJ, Cowan BJ, et al. Pannexin 1 and pannexin 3 are glycoproteins that exhibit many distinct characteristics from the connexin family of gap junction proteins. J Cell Sci. 2007;120:3772–83.
Yu G, Wu X, Kilroy G, Halvorsen YD, Gimble JM, Floyd ZE. Isolation of murine adipose-derived stem cells. Methods Mol Biol. 2011;702:29–36.
Gao M, Ma Y, Liu D. High-fat diet-induced adiposity, adipose inflammation, hepatic steatosis and hyperinsulinemia in outbred CD-1 mice. PLoS One. 2015;10:e0119784.
Abitbol JM, O’Donnell BL, Wakefield CB, Jewlal E, Kelly JJ, Barr K, et al. Double deletion of Panx1 and Panx3 affects skin and bone but not hearing. J Mol Med (Berl). 2019;97:723–36.
Celetti SJ, Cowan KN, Penuela S, Shao Q, Churko J, Laird DW. Implications of pannexin 1 and pannexin 3 for keratinocyte differentiation. J Cell Sci. 2010;123:1363–72.
Zhang P, Ishikawa M, Doyle A, Nakamura T, He B, Yamada Y. Pannexin 3 regulates skin development via Epiprofin. Sci Rep. 2021;11:1779.
Zhang P, Ishikawa M, Rhodes C, Doyle A, Ikeuchi T, Nakamura K, et al. Pannexin-3 deficiency delays skin wound healing in mice due to defects in channel functionality. J Invest Dermatol. 2019;139:909–18.
Bond SR, Lau A, Penuela S, Sampaio AV, Underhill TM, Laird DW, et al. Pannexin 3 is a novel target for Runx2, expressed by osteoblasts and mature growth plate chondrocytes. J Bone Miner Res. 2011;26:2911–22.
Ishikawa M, Williams G, Forcinito P, Ishikawa M, Petrie RJ, Saito K, et al. Pannexin 3 ER Ca(2+) channel gating is regulated by phosphorylation at the Serine 68 residue in osteoblast differentiation. Sci Rep. 2019;9:18759.
Iwamoto T, Nakamura T, Doyle A, Ishikawa M, de Vega S, Fukumoto S, et al. Pannexin 3 regulates intracellular ATP/cAMP levels and promotes chondrocyte differentiation. J Biol Chem. 2010;285:18948–58.
Moon PM, Shao ZY, Wambiekele G, Appleton C, Laird DW, Penuela S, et al. Global deletion of pannexin 3 accelerates development of aging-induced osteoarthritis in mice. Arthritis Rheumatol. 2021;73:1178–88.
Link JC, Reue K. Genetic basis for sex differences in obesity and lipid metabolism. Annu Rev Nutr. 2017;37:225–45.
Ingvorsen C, Karp NA, Lelliott CJ. The role of sex and body weight on the metabolic effects of high-fat diet in C57BL/6N mice. Nutr Diabetes. 2017;7:e261.
Shin JH, Hur JY, Seo HS, Jeong YA, Lee JK, Oh MJ, et al. The ratio of estrogen receptor alpha to estrogen receptor beta in adipose tissue is associated with leptin production and obesity. Steroids. 2007;72:592–9.
Turmel P, Dufresne J, Hermo L, Smith CE, Penuela S, Laird DW, et al. Characterization of pannexin1 and pannexin3 and their regulation by androgens in the male reproductive tract of the adult rat. Mol Reprod Dev. 2011;78:124–38.
Varghese M, Griffin C, McKernan K, Eter L, Lanzetta N, Agarwal D, et al. Sex differences in inflammatory responses to adipose tissue lipolysis in diet-induced obesity. Endocrinology. 2019;160:293–312.
Winn NC, Cottam MA, Wasserman DH, Hasty AH. Exercise and adipose tissue immunity: outrunning inflammation. Obesity. 2021;29:790–801.
Paolucci EM, Loukov D, Bowdish DME, Heisz JJ. Exercise reduces depression and inflammation but intensity matters. Biol Psychol. 2018;133:79–84.
Petersen AM, Pedersen BK. The anti-inflammatory effect of exercise. J Appl Physiol. 2005;98:1154–62. (1985)
Suzuki K. Chronic inflammation as an immunological abnormality and effectiveness of exercise. Biomolecules. 2019;9:223.
Rosa-Neto JC, Silveira LS. Endurance exercise mitigates immunometabolic adipose tissue disturbances in cancer and obesity. Int J Mol Sci. 2020;21:9745.
Kawanishi N, Yano H, Yokogawa Y, Suzuki K. Exercise training inhibits inflammation in adipose tissue via both suppression of macrophage infiltration and acceleration of phenotypic switching from M1 to M2 macrophages in high-fat-diet-induced obese mice. Exerc Immunol Rev. 2010;16:105–18.
Swift DL, Johannsen NM, Lavie CJ, Earnest CP, Church TS. The role of exercise and physical activity in weight loss and maintenance. Prog Cardiovasc Dis. 2014;56:441–7.
Swift DL, McGee JE, Earnest CP, Carlisle E, Nygard M, Johannsen NM. The effects of exercise and physical activity on weight loss and maintenance. Prog Cardiovasc Dis. 2018;61:206–13.
Carpenter KC, Strohacker K, Breslin WL, Lowder TW, Agha NH, McFarlin BK. Effects of exercise on weight loss and monocytes in obese mice. Comp Med. 2012;62:21–6.
McCabe LR, Irwin R, Tekalur A, Evans C, Schepper JD, Parameswaran N, et al. Exercise prevents high fat diet-induced bone loss, marrow adiposity and dysbiosis in male mice. Bone. 2019;118:20–31.
Golay A, Bobbioni E. The role of dietary fat in obesity. Int J Obes Relat Metab Disord. 1997;21:S2–11.
Wang L, Wang H, Zhang B, Popkin BM, Du S. Elevated fat intake increases body weight and the risk of overweight and obesity among chinese adults: 1991-2015 trends. Nutrients. 2020;12:3272.
Levine JA, Eberhardt NL, Jensen MD. Role of nonexercise activity thermogenesis in resistance to fat gain in humans. Science. 1999;283:212–4.
Ajuwon KM, Spurlock ME. Palmitate activates the NF-kappaB transcription factor and induces IL-6 and TNFalpha expression in 3T3-L1 adipocytes. J Nutr. 2005;135:1841–6.
Cavalcante PAM, Gregnani MF, Henrique JS, Ornellas FH, Araújo RC. Aerobic but not resistance exercise can induce inflammatory pathways via toll-like 2 and 4: a systematic review. Sports Med Open. 2017;3:42.
Acknowledgements
We thank the funding agencies that supported this work including: Petro-Canada Young Innovator Award – Western University to SP, Ontario Graduate Scholarship to CBW. FB holds the Canada Research Chair in Musculoskeletal Research and is the recipient of a Foundation Grant from the Canadian Institutes of Health Research (CIHR, Grant #332438). CIHR Foundation Grant (FRN:FDN-143203) to AK. SP is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Author information
Authors and Affiliations
Contributions
CBW: project design, mouse husbandry, research data, data analysis, wrote manuscript; VRL: research data, data analysis, edited manuscript; DJ: research data, reviewed manuscript; PB: research data, data analysis; NJP: research data, data analysis, edited manuscript; SS, BLOD, JT, and RES-P: research data, data analysis, edited manuscript; KJB: research data, mouse husbandry, reviewed and edited manuscript; RG: research data, metabolic cage analysis; LF: research data, edited manuscript; NBM: research data, edited manuscript; AK: research data analysis, manuscript review and editing; FB: project design, manuscript review and editing, funding; SP: project design and supervision, data analysis, funding, manuscript review and editing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Wakefield, C.B., Lee, V.R., Johnston, D. et al. Pannexin 3 deletion reduces fat accumulation and inflammation in a sex-specific manner. Int J Obes 46, 726–738 (2022). https://doi.org/10.1038/s41366-021-01037-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41366-021-01037-4
This article is cited by
-
Associations of Dietary Anthocyanidins Intake with Bone Health in Children: A Cross-Sectional Study
Calcified Tissue International (2023)