Abstract
Background
Dietary bioactive compounds have been demonstrated to produce several health benefits. Genistein, an isoflavone of soy protein, and resveratrol, a polyphenol from grapes, have been shown to improve insulin sensitivity and to stimulate white adipose tissue (WAT) browning, leading to increased energy expenditure. However, it has not been demonstrated in humans whether genistein or resveratrol have the capacity to stimulate the differentiation of stromal vascular fraction (SVF) cells from white fat into beige adipocytes.
Subjects/methods
With this aim, we assessed whether stromal vascular fraction cells obtained from biopsies of the subdermal fat depots of subjects with normal body weight (NW) or from subjects with overweight/obesity with (OIR) or without (OIS) insulin resistance were able to differentiate into the beige adipose tissue lineage in vitro, by exposing the cells to genistein, resveratrol, or the combination of both.
Results
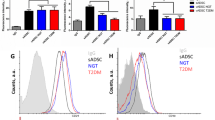
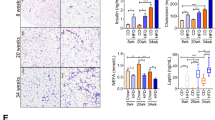
The results showed that SVF cells obtained from NW or OIS subjects were able to differentiate into beige adipocytes according to an increased expression of beige biomarkers including UCP1, PDRM-16, PGC1α, CIDEA, and SHOX2 upon exposure to genistein. However, SVF cells from OIR subjects were unable to differentiate into beige adipocytes with any of the inducers. Exposure to resveratrol or the combination of resveratrol/genistein did not significantly stimulate the expression of browning markers in any of the groups studied. We found that the non-responsiveness of the SVF from subjects with obesity and insulin resistance to any of the inducers was associated with an increase in the expression of endoplasmic reticulum stress markers.
Conclusion
Consumption of genistein may stimulate WAT browning mainly in NW or OIS subjects. Thus, obesity associated with insulin resistance may be considered as a condition that prevents some beneficial effects of some dietary bioactive compounds.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Change history
09 March 2022
A Correction to this paper has been published: https://doi.org/10.1038/s41366-022-01104-4
References
Taylor VH, Forhan M, Vigod SN, McIntyre RS, Morrison KM. The impact of obesity on quality of life. Best Pract Res Clin Endocrinol Metab. 2013;27:139–46.
Choe SS, Huh JY, Hwang IJ, Kim JI, Kim JB. Adipose tissue remodeling: its role in energy metabolism and metabolic disorders. Front Endocrinol. 2016;7:30.
Sethi JK, Vidal-Puig AJ. Thematic review series: adipocyte biology. Adipose tissue function and plasticity orchestrate nutritional adaptation. J Lipid Res. 2007;48:1253–62.
Vargas-Castillo A, Fuentes-Romero R, Rodriguez-Lopez LA, Torres N, Tovar AR. Understanding the biology of thermogenic fat: is browning a new approach to the treatment of obesity? Arch Med Res. 2017;48:401–13.
Barbatelli G, Murano I, Madsen L, Hao Q, Jimenez M, Kristiansen K, et al. The emergence of cold-induced brown adipocytes in mouse white fat depots is determined predominantly by white to brown adipocyte transdifferentiation. Am J Physiol Endocrinol Metab. 2010;298:E1244–53.
Wu J, Cohen P, Spiegelman BM. Adaptive thermogenesis in adipocytes: is beige the new brown? Genes Dev. 2013;27:234–50.
Granneman JG, Burnazi M, Zhu Z, Schwamb LA. White adipose tissue contributes to UCP1-independent thermogenesis. Am J Physiol Endocrinol Metab. 2003;285:E1230–6.
Ikeda K, Kang Q, Yoneshiro T, Camporez JP, Maki H, Homma M, et al. UCP1-independent signaling involving SERCA2b-mediated calcium cycling regulates beige fat thermogenesis and systemic glucose homeostasis. Nat Med. 2017;23:1454–65.
Nedergaard J, Cannon B. The browning of white adipose tissue: some burning issues. Cell Metab. 2014;20:396–407.
Wang W, Seale P. Control of brown and beige fat development. Nat Rev Mol Cell Biol. 2016;17:691–702.
Wu Y, Kinnebrew MA, Kutyavin VI, Chawla A. Distinct signaling and transcriptional pathways regulate peri-weaning development and cold-induced recruitment of beige adipocytes. Proc Natl Acad Sci U S A. 2020;117:6883–9.
Kim HJ, Choi EJ, Kim HS, Choi CW, Choi SW, Kim SL, et al. Germinated soy germ extract ameliorates obesity through beige fat activation. Food Funct. 2019;10:836–48.
Wu L, Zhang L, Li B, Jiang H, Duan Y, Xie Z, et al. AMP-activated protein kinase (AMPK) regulates energy metabolism through modulating thermogenesis in adipose tissue. Front Physiol. 2018;9:122.
Zou T, Wang B, Li S, Liu Y, You J. Dietary apple polyphenols promote fat browning in high-fat diet-induced obese mice through activation of adenosine monophosphate-activated protein kinase alpha. J Sci Food Agric. 2020;100:2389–98.
Bartelt A, Heeren J. Adipose tissue browning and metabolic health. Nat Rev Endocrinol. 2014;10:24–36.
Fernandez-Verdejo R, Marlatt KL, Ravussin E, Galgani JE. Contribution of brown adipose tissue to human energy metabolism. Mol Aspects Med. 2019;68:82–89.
Longo M, Spinelli R, D’Esposito V, Zatterale F, Fiory F, Nigro C, et al. Pathologic endoplasmic reticulum stress induced by glucotoxic insults inhibits adipocyte differentiation and induces an inflammatory phenotype. Biochim Biophys Acta. 2016;1863:1146–56.
Shimada T, Hiramatsu N, Okamura M, Hayakawa K, Kasai A, Yao J, et al. Unexpected blockade of adipocyte differentiation by K-7174: implication for endoplasmic reticulum stress. Biochem Biophys Res Commun. 2007;363:355–60.
Cnop M, Foufelle F, Velloso LA. Endoplasmic reticulum stress, obesity and diabetes. Trends Mol Med. 2012;18:59–68.
Ozcan U, Cao Q, Yilmaz E, Lee AH, Iwakoshi NN, Ozdelen E, et al. Endoplasmic reticulum stress links obesity, insulin action, and type 2 diabetes. Science. 2004;306:457–61.
Grossini E, Farruggio S, Raina G, Mary D, Deiro G, Gentilli S. Effects of genistein on differentiation and viability of human visceral adipocytes. Nutrients. 2018;10:1–18.
Wang S, Liang X, Yang Q, Fu X, Rogers CJ, Zhu M, et al. Resveratrol induces brown-like adipocyte formation in white fat through activation of AMP-activated protein kinase (AMPK) alpha1. Int J Obes. 2015;39:967–76.
Zou T, Chen D, Yang Q, Wang B, Zhu MJ, Nathanielsz PW, et al. Resveratrol supplementation of high-fat diet-fed pregnant mice promotes brown and beige adipocyte development and prevents obesity in male offspring. J Physiol. 2017;595:1547–62.
Mendez-del Villar M, Gonzalez-Ortiz M, Martinez-Abundis E, Perez-Rubio KG, Lizarraga-Valdez R. Effect of resveratrol administration on metabolic syndrome, insulin sensitivity, and insulin secretion. Metab Syndr Relat Disord. 2014;12:497–501.
Konings E, Timmers S, Boekschoten MV, Goossens GH, Jocken JW, Afman LA, et al. The effects of 30 days resveratrol supplementation on adipose tissue morphology and gene expression patterns in obese men. Int J Obes. 2014;38:470–3.
Lan F, Weikel KA, Cacicedo JM, Ido Y. Resveratrol-induced AMP-activated protein kinase activation is cell-type dependent: lessons from basic research for clinical application. Nutrients. 2017;9:1–14.
Carey AL, Vorlander C, Reddy-Luthmoodoo M, Natoli AK, Formosa MF, Bertovic DA, et al. Reduced UCP-1 content in in vitro differentiated beige/brite adipocytes derived from preadipocytes of human subcutaneous white adipose tissues in obesity. PLoS ONE. 2014;9:e91997.
Guilherme A, Virbasius JV, Puri V, Czech MP. Adipocyte dysfunctions linking obesity to insulin resistance and type 2 diabetes. Nat Rev Mol Cell Biol. 2008;9:367–77.
Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–9.
World Health Organization. Obesity: preventing and managing the global epidemic. Report of a WHO consultation. World Health Organization Technical Report Series, vol. 894. World Health Organization. Geneva, Switzerland; 2000. p. i–xii, 1–253.
Mehlem A, Hagberg CE, Muhl L, Eriksson U, Falkevall A. Imaging of neutral lipids by oil red O for analyzing the metabolic status in health and disease. Nat Protoc. 2013;8:1149–54.
Galgani JE, Moro C, Ravussin E. Metabolic flexibility and insulin resistance. Am J Physiol Endocrinol Metab. 2008;295:E1009–17.
Wosnitza M, Hemmrich K, Groger A, Graber S, Pallua N. Plasticity of human adipose stem cells to perform adipogenic and endothelial differentiation. Differentiation. 2007;75:12–23.
Loh RKC, Formosa MF, Eikelis N, Bertovic DA, Anderson MJ, Barwood SA, et al. Pioglitazone reduces cold-induced brown fat glucose uptake despite induction of browning in cultured human adipocytes: a randomised, controlled trial in humans. Diabetologia. 2018;61:220–30.
Palacios-Gonzalez B, Vargas-Castillo A, Velazquez-Villegas LA, Vasquez-Reyes S, Lopez P, Noriega LG, et al. Genistein increases the thermogenic program of subcutaneous WAT and increases energy expenditure in mice. J Nutr Biochem. 2019;68:59–68.
Yuliana A, Daijo A, Jheng HF, Kwon J, Nomura W, Takahashi H, et al. Endoplasmic reticulum stress impaired uncoupling protein 1 expression via the suppression of peroxisome proliferator-activated receptor gamma binding activity in mice beige adipocytes. Int J Mol Sci. 2019;20:1–15.
Cuthbertson DJ, Steele T, Wilding JP, Halford JC, Harrold JA, Hamer M, et al. What have human experimental overfeeding studies taught us about adipose tissue expansion and susceptibility to obesity and metabolic complications? Int J Obes. 2017;41:853–65.
Kyrou I, Chrousos GP, Tsigos C. Stress, visceral obesity, and metabolic complications. Ann N Y Acad Sci. 2006;1083:77–110.
Kahn SE, Hull RL, Utzschneider KM. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature. 2006;444:840–6.
Karpe F, Dickmann JR, Frayn KN. Fatty acids, obesity, and insulin resistance: time for a reevaluation. Diabetes. 2011;60:2441–9.
Qatanani M, Lazar MA. Mechanisms of obesity-associated insulin resistance: many choices on the menu. Genes Dev. 2007;21:1443–55.
Vazquez-Vela ME, Torres N, Tovar AR. White adipose tissue as endocrine organ and its role in obesity. Arch Med Res. 2008;39:715–28.
Hutley LJ, Herington AC, Shurety W, Cheung C, Vesey DA, Cameron DP, et al. Human adipose tissue endothelial cells promote preadipocyte proliferation. Am J Physiol Endocrinol Metab. 2001;281:E1037–44.
Rodriguez AM, Elabd C, Delteil F, Astier J, Vernochet C, Saint-Marc P, et al. Adipocyte differentiation of multipotent cells established from human adipose tissue. Biochem Biophys Res Commun. 2004;315:255–63.
Kajimura S, Spiegelman BM, Seale P. Brown and beige fat: physiological roles beyond heat generation. Cell Metab. 2015;22:546–59.
Shabalina IG, Petrovic N, de Jong JM, Kalinovich AV, Cannon B, Nedergaard J. UCP1 in brite/beige adipose tissue mitochondria is functionally thermogenic. Cell Rep. 2013;5:1196–203.
Azhar Y, Parmar A, Miller CN, Samuels JS, Rayalam S. Phytochemicals as novel agents for the induction of browning in white adipose tissue. Nutr Metab. 2016;13:89.
Arzola-Paniagua MA, Garcia-Salgado Lopez ER, Calvo-Vargas CG, Guevara-Cruz M. Efficacy of an orlistat-resveratrol combination for weight loss in subjects with obesity: a randomized controlled trial. Obesity. 2016;24:1454–63.
Faghihzadeh F, Adibi P, Rafiei R, Hekmatdoost A. Resveratrol supplementation improves inflammatory biomarkers in patients with nonalcoholic fatty liver disease. Nutr Res. 2014;34:837–43.
Poulsen MM, Vestergaard PF, Clasen BF, Radko Y, Christensen LP, Stodkilde-Jorgensen H, et al. High-dose resveratrol supplementation in obese men: an investigator-initiated, randomized, placebo-controlled clinical trial of substrate metabolism, insulin sensitivity, and body composition. Diabetes. 2013;62:1186–95.
Timmers S, Konings E, Bilet L, Houtkooper RH, van de Weijer T, Goossens GH, et al. Calorie restriction-like effects of 30 days of resveratrol supplementation on energy metabolism and metabolic profile in obese humans. Cell Metab. 2011;14:612–22.
Chachay VS, Macdonald GA, Martin JH, Whitehead JP, O’Moore-Sullivan TM, Lee P, et al. Resveratrol does not benefit patients with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol. 2014;12:2092–103.e1–6.
Yoshino J, Conte C, Fontana L, Mittendorfer B, Imai S, Schechtman KB, et al. Resveratrol supplementation does not improve metabolic function in nonobese women with normal glucose tolerance. Cell Metab. 2012;16:658–64.
Alberdi G, Rodriguez VM, Miranda J, Macarulla MT, Churruca I, Portillo MP. Thermogenesis is involved in the body-fat lowering effects of resveratrol in rats. Food Chem. 2013;141:1530–5.
Asnani-Kishnani M, Rodriguez AM, Serrano A, Palou A, Bonet ML, Ribot J. Neonatal resveratrol and nicotinamide riboside supplementations sex-dependently affect beige transcriptional programming of preadipocytes in mouse adipose tissue. Front Physiol. 2019;10:83.
Aziz SA, Wakeling LA, Miwa S, Alberdi G, Hesketh JE, Ford D. Metabolic programming of a beige adipocyte phenotype by genistein. Mol Nutr Food Res. 2017;61:1–10.
Zhang M, Ikeda K, Xu JW, Yamori Y, Gao XM, Zhang BL. Genistein suppresses adipogenesis of 3T3-L1 cells via multiple signal pathways. Phytother Res. 2009;23:713–8.
Contreras C, Gonzalez-Garcia I, Seoane-Collazo P, Martinez-Sanchez N, Linares-Pose L, Rial-Pensado E, et al. Reduction of hypothalamic endoplasmic reticulum stress activates browning of white fat and ameliorates obesity. Diabetes. 2017;66:87–99.
Onate B, Vilahur G, Ferrer-Lorente R, Ybarra J, Diez-Caballero A, Ballesta-Lopez C, et al. The subcutaneous adipose tissue reservoir of functionally active stem cells is reduced in obese patients. FASEB J. 2012;26:4327–36.
Zimmerlin L, Donnenberg VS, Pfeifer ME, Meyer EM, Peault B, Rubin JP, et al. Stromal vascular progenitors in adult human adipose tissue. Cytometry A. 2010;77:22–30.
Banyard DA, Sarantopoulos CN, Borovikova AA, Qiu X, Wirth GA, Paydar KZ, et al. Phenotypic analysis of stromal vascular fraction after mechanical shear reveals stress-induced progenitor populations. Plast Reconstr Surg. 2016;138:237e–47e.
Kim WS, Park BS, Kim HK, Park JS, Kim KJ, Choi JS, et al. Evidence supporting antioxidant action of adipose-derived stem cells: protection of human dermal fibroblasts from oxidative stress. J Dermatol Sci. 2008;49:133–42.
Torre-Villalvazo I, Bunt AE, Aleman G, Marquez-Mota CC, Diaz-Villasenor A, Noriega LG, et al. Adiponectin synthesis and secretion by subcutaneous adipose tissue is impaired during obesity by endoplasmic reticulum stress. J Cell Biochem. 2018;119:5970–84.
Boden G, Duan X, Homko C, Molina EJ, Song W, Perez O, et al. Increase in endoplasmic reticulum stress-related proteins and genes in adipose tissue of obese, insulin-resistant individuals. Diabetes. 2008;57:2438–44.
Hotamisligil GS. Inflammation and endoplasmic reticulum stress in obesity and diabetes. Int J Obes. 2008;32 Suppl 7:S52–4.
Sage AT, Holtby-Ottenhof S, Shi Y, Damjanovic S, Sharma AM, Werstuck GH. Metabolic syndrome and acute hyperglycemia are associated with endoplasmic reticulum stress in human mononuclear cells. Obesity. 2012;20:748–55.
Acknowledgements
This work was supported by the National Council of Science and Technology Mexico (CONACYT) (Grant Number 261843 to ART) and supported by the National Institute of Medical Sciences and Nutrition S.Z. Leonardo Rodriguez-López received a CONACYT scholarship for the Basic Biomedical Research program (Investigacion Biomedica Basica), UNAM.
Author information
Authors and Affiliations
Contributions
LAR-L: experimental work and analysis; IT-V: experimental design; GA-E: experimental work and writing; AF-L and MG-C: clinical and experimental work; MS-T, EAT-A, VM-L, and SV-R: experimental work; GMT-V: surgical process; YM: data analysis; NT: experimental design and writing; ART: experimental concept, design, analysis, and writing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Rodriguez-López, L.A., Torre-Villalvazo, I., Aleman-Escondrillas, G. et al. The capacity of differentiation of stromal vascular fraction cells into beige adipocytes is markedly reduced in subjects with overweight/obesity and insulin resistance: effect of genistein. Int J Obes 45, 2471–2481 (2021). https://doi.org/10.1038/s41366-021-00921-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41366-021-00921-3