Abstract
Objective
Recent evidence indicates that levels of breast milk (BM) hormones such as leptin can fluctuate with maternal adiposity, suggesting that BM hormones may signal maternal metabolic and nutritional environments to offspring during postnatal development. The hormone apelin is highly abundant in BM but its regulation during lactation is completely unknown. Here, we evaluated whether maternal obesity and overnutrition impacted BM apelin and leptin levels in clinical cohorts and lactating rats.
Methods
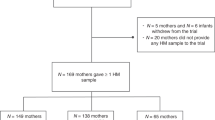
BM and plasma samples were collected from normal-weight and obese breastfeeding women, and from lactating rats fed a control or a high fat (HF) diet during lactation. Apelin and leptin levels were assayed by ELISA. Mammary gland (MG) apelin expression and its cellular localization in lactating rats was measured by quantitative RT-PCR and immunofluorescence, respectively.
Results
BM apelin levels increased with maternal BMI, whereas plasma apelin levels decreased. BM apelin was also positively correlated with maternal insulin and C-peptide levels. In rats, maternal HF feeding exclusively during lactation was sufficient to increase BM apelin levels and decrease its plasma concentration without changing body weight. In contrast, BM leptin levels increased with maternal BMI in humans, but did not change with maternal HF feeding during lactation in rats. Apelin is highly expressed in the rat MG during lactation and was mainly localized to mammary myoepithelial cells. We found that MG apelin gene expression was up-regulated by maternal HF diet and positively correlated with BM apelin content and maternal insulinemia.
Conclusions
Our study indicates that BM apelin levels increase with long- and short-term overnutrition, possibly via maternal hyperinsulinemia and transcriptional upregulation of MG apelin expression in myoepithelial cells. Apelin regulates many physiological processes, including energy metabolism, digestive function, and development. Further studies are needed to unravel the consequences of such changes in offspring development.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Badillo-Suarez PA, Rodriguez-Cruz M, Nieves-Morales X. Impact of metabolic hormones secreted in human breast milk on nutritional programming in childhood obesity. J Mammary Gland Biol Neoplasia. 2017;22:171–91.
Eriksen KG, Christensen SH, Lind MV, Michaelsen KF. Human milk composition and infant growth. Curr Opin Clin Nutr Metabolic Care. 2018;21:200–6.
Kratzsch J, Bae YJ, Kiess W. Adipokines in human breast milk. Best Pract Res Clin Endocrinol Metab. 2018;32:27–38.
Palou A, Pico C. Leptin intake during lactation prevents obesity and affects food intake and food preferences in later life. Appetite. 2009;52:249–52.
Innis SM. Impact of maternal diet on human milk composition and neurological development of infants. Am J Clin Nutr. 2014;99:734S–41S.
Andreas NJ, Hyde MJ, Gale C, Parkinson JR, Jeffries S, Holmes E, et al. Effect of maternal body mass index on hormones in breast milk: a systematic review. PloS One. 2014;9:e115043.
Demmelmair H, Koletzko B. Variation of metabolite and hormone contents in human milk. Clin Perinatol. 2017;44:151–64.
Isganaitis E, Venditti S, Matthews TJ, Lerin C, Demerath EW, Fields DA. Maternal obesity and the human milk metabolome: associations with infant body composition and postnatal weight gain. Am J Clin Nutr. 2019;110:111–20.
Plagemann A, Harder T, Rodekamp E, Dudenhausen JW. Breast-feeding and risk for childhood obesity: response to Mayer-Davis et al. Diabetes Care. 2007;30:451–2. author reply 452
Kramer MS, Oken E, Martin RM. Infant feeding and adiposity: scientific challenges in life-course epidemiology. Am J Clin Nutr. 2014;99:1281–3.
Fields DA, George B, Williams M, Whitaker K, Allison DB, Teague A, et al. Associations between human breast milk hormones and adipocytokines and infant growth and body composition in the first 6 months of life. Pediatr Obes. 2017;12 Suppl 1:78–85.
Habata Y, Fujii R, Hosoya M, Fukusumi S, Kawamata Y, Hinuma S, et al. Apelin, the natural ligand of the orphan receptor APJ, is abundantly secreted in the colostrum. Biochimica et biophysica acta. 1999;1452:25–35.
Aydin S. The presence of the peptides apelin, ghrelin and nesfatin-1 in the human breast milk, and the lowering of their levels in patients with gestational diabetes mellitus. Peptides. 2010;31:2236–40.
Mesmin C, Fenaille F, Becher F, Tabet JC, Ezan E. Identification and characterization of apelin peptides in bovine colostrum and milk by liquid chromatography-mass spectrometry. J Proteome Res. 2011;10:5222–31.
Wysocka MB, Pietraszek-Gremplewicz K, Nowak D. The role of apelin in cardiovascular diseases, obesity and cancer. Front Physiol. 2018;9:557.
Eberle D, Marousez L, Hanssens S, Knauf C, Breton C, Deruelle P. et al. Elabela and Apelin actions in healthy and pathological pregnancies. Cytokine Growth Factor Rev. 2019;46:45–53.
Huang Z, Luo X, Liu M, Chen L. Function and regulation of apelin/APJ system in digestive physiology and pathology. J Cell Physiol. 2019;234:7796–810.
Kawamata Y, Habata Y, Fukusumi S, Hosoya M, Fujii R, Hinuma S, et al. Molecular properties of apelin: tissue distribution and receptor binding. Biochimica et biophysica acta. 2001;1538:162–71.
Hosoya M, Kawamata Y, Fukusumi S, Fujii R, Habata Y, Hinuma S, et al. Molecular and functional characteristics of APJ. Tissue distribution of mRNA and interaction with the endogenous ligand apelin. J Biological Chem. 2000;275:21061–7.
Boucher J, Masri B, Daviaud D, Gesta S, Guigne C, Mazzucotelli A, et al. Apelin, a newly identified adipokine up-regulated by insulin and obesity. Endocrinology. 2005;146:1764–71.
Castan-Laurell I, Dray C, Attane C, Duparc T, Knauf C, Valet P. Apelin, diabetes, and obesity. Endocrine. 2011;40:1–9.
Hanssens S, Marx-Deseure A, Lecoutre S, Butruille L, Fournel A, Knauf C, et al. Maternal obesity alters the apelinergic system at the feto-maternal interface. Placenta. 2016;39:41–4.
Zaki M, Kamal S, Ezzat W, Hassan N, Yousef W, Ryad H, et al. Serum apelin levels and metabolic risk markers in obese women. J Genet Eng Biotechnol. 2017;15:423–9.
Butruille L, Marousez L, Pourpe C, Oger F, Lecoutre S, Catheline D, et al. Maternal high-fat diet during suckling programs visceral adiposity and epigenetic regulation of adipose tissue stearoyl-CoA desaturase-1 in offspring. Int J Obes. 2019;43:2381–93.
Whitmore TJ, Trengove NJ, Graham DF, Hartmann PE. Analysis of insulin in human breast milk in mothers with type 1 and type 2 diabetes mellitus. Int J Endocrinol. 2012;2012:296368.
Casabiell X, Pineiro V, Tome MA, Peino R, Dieguez C, Casanueva FF. Presence of leptin in colostrum and/or breast milk from lactating mothers: a potential role in the regulation of neonatal food intake. J Clin Endocrinol Metab. 1997;82:4270–3.
Young BE, Patinkin Z, Palmer C, de la Houssaye B, Barbour LA, Hernandez T, et al. Human milk insulin is related to maternal plasma insulin and BMI: but other components of human milk do not differ by BMI. Eur J Clin Nutr. 2017;71:1094–1100.
Houseknecht KL, McGuire MK, Portocarrero CP, McGuire MA, Beerman K. Leptin is present in human milk and is related to maternal plasma leptin concentration and adiposity. Biochem Biophys Res Commun. 1997;240:742–7.
Uysal FK, Onal EE, Aral YZ, Adam B, Dilmen U, Ardicolu Y. Breast milk leptin: its relationship to maternal and infant adiposity. Clin Nutr. 2002;21:157–60.
Kucur M, Tuten A, Oncul M, Acikgoz AS, Yuksel MA, Imamoglu M, et al. Maternal serum apelin and YKL-40 levels in early and late-onset pre-eclampsia. Hypertens Pregnancy. 2014;33:467–75.
Wang Z, Greeley GH Jr, Qiu S. Immunohistochemical localization of apelin in human normal breast and breast carcinoma. J Mol Histol. 2008;39:121–4.
Gronberg M, Amini RM, Stridsberg M, Janson ET, Saras J. Neuroendocrine markers are expressed in human mammary glands. Regul Pept. 2010;160:68–74.
Mercati F, Maranesi M, Dall'Aglio C, Petrucci L, Pasquariello R, Tardella FM, et al. Apelin system in mammary gland of sheep reared in semi-natural pastures of the central apennines. Animals. 2018;8:223.
Macias H, Hinck L. Mammary gland development. Wiley Interdiscip Rev Dev Biol. 2012;1:533–57.
Barsky SH, Karlin NJ. Myoepithelial cells: autocrine and paracrine suppressors of breast cancer progression. J Mammary Gland Biol Neoplasia. 2005;10:249–60.
Bonnet M, Gourdou I, Leroux C, Chilliard Y, Djiane J. Leptin expression in the ovine mammary gland: putative sequential involvement of adipose, epithelial, and myoepithelial cells during pregnancy and lactation. J Anim Sci. 2002;80:723–8.
Jarde T, Caldefie-Chezet F, Damez M, Mishellany F, Perrone D, Penault-Llorca F, et al. Adiponectin and leptin expression in primary ductal breast cancer and in adjacent healthy epithelial and myoepithelial tissue. Histopathology. 2008;53:484–7.
Wei L, Hou X, Tatemoto K. Regulation of apelin mRNA expression by insulin and glucocorticoids in mouse 3T3-L1 adipocytes. Regul Pept. 2005;132:27–32.
Burnol AF, Loizeau M, Girard J. Insulin receptor activity and insulin sensitivity in mammary gland of lactating rats. Am J Physiol. 1990;259:E828–34.
Hvid H, Fels JJ, Kirk RK, Thorup I, Jensen HE, Hansen BF, et al. In situ phosphorylation of Akt and ERK1/2 in rat mammary gland, colon, and liver following treatment with human insulin and IGF-1. Toxicol Pathol. 2011;39:623–40.
Perez-Echarri N, Perez-Matute P, Marcos-Gomez B, Martinez JA, Moreno-Aliaga MJ. Effects of eicosapentaenoic acid ethyl ester on visfatin and apelin in lean and overweight (cafeteria diet-fed) rats. Br J Nutr. 2009;101:1059–67.
Lorente-Cebrian S, Bustos M, Marti A, Martinez JA, Moreno-Aliaga MJ. Eicosapentaenoic acid up-regulates apelin secretion and gene expression in 3T3-L1 adipocytes. Mol Nutr Food Res. 2010;54 Suppl 1:S104–11.
Prostek A, Gajewska M, Balasinska B. The influence of eicosapentaenoic acid and docosahexaenoic acid on expression of genes connected with metabolism and secretory functions of ageing 3T3-L1 adipocytes. Prostaglandins Other Lipid Mediat. 2016;125:48–56.
Bertrand C, Pignalosa A, Wanecq E, Rancoule C, Batut A, Deleruyelle S, et al. Effects of dietary eicosapentaenoic acid (EPA) supplementation in high-fat fed mice on lipid metabolism and apelin/APJ system in skeletal muscle. PloS One. 2013;8:e78874.
Guo W, Liu J, Hou S, Hu G, Ma H, Gong Q, et al. The inflammatory environment mediated by a high-fat diet inhibited the development of mammary glands and destroyed the tight junction in pregnant mice. Food Funct. 2020;11:8193–201.
Galon-Tilleman H, Yang H, Bednarek MA, Spurlock SM, Paavola KJ, Ko B, et al. Apelin-36 modulates blood glucose and body weight independently of canonical APJ receptor signaling. J Biological Chem. 2017;292:1925–33.
Palou M, Pico C, Palou A. Leptin as a breast milk component for the prevention of obesity. Nutr Rev. 2018;76:875–92.
Gavalda-Navarro A, Hondares E, Giralt M, Mampel T, Iglesias R, Villarroya F. Fibroblast growth factor 21 in breast milk controls neonatal intestine function. Sci Rep. 2015;5:13717.
Buts JP, De Keyser N, Dive C. Intestinal development in the suckling rat: effect of insulin on the maturation of villus and crypt cell functions. Eur J Clin Invest. 1988;18:391–8.
Lambrecht NW, Yakubov I, Zer C, Sachs G. Transcriptomes of purified gastric ECL and parietal cells: identification of a novel pathway regulating acid secretion. Physiol Genomics. 2006;25:153–65.
Wang G, Kundu R, Han S, Qi X, Englander EW, Quertermous T, et al. Ontogeny of apelin and its receptor in the rodent gastrointestinal tract. Regul Pept. 2009;158:32–9.
Antushevich H, Bierla J, Pawlina B, Kapica M, Krawczynska A, Herman AP, et al. Apelin’s effects on young rat gastrointestinal tract maturation. Peptides. 2015;65:1–5.
Fournel A, Drougard A, Duparc T, Marlin A, Brierley SM, Castro J, et al. Apelin targets gut contraction to control glucose metabolism via the brain. Gut. 2017;66:258–69.
Dray C, Sakar Y, Vinel C, Daviaud D, Masri B, Garrigues L, et al. The intestinal glucose-apelin cycle controls carbohydrate absorption in mice. Gastroenterology. 2013;144:771–80.
Marousez L, Lesage J, Eberle D. Epigenetics: linking early postnatal nutrition to obesity programming? Nutrients. 2019;11:2966.
Acknowledgements
We thank Valérie Montel, Anne Dickes and Phexmar animal housing facility for excellent help and support in animal studies; Elodie Richard at BICeL facility for microscopy and Barbara Deracinois for advice in MG peptides extractions.
Funding
This study was supported by grants of the French Ministry of Higher Education and Research, Lille University (BQR 2014), the FHU 1000 days for health (APELMILK project), the CHRU Lille hospital (OBAPE project) and Conseil Régional des Hauts-de-France. L.M. was supported by fellowships from Metropole Européenne Lilloise (MEL) and Conseil Régional des Hauts-de-France.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Marousez, L., Hanssens, S., Butruille, L. et al. Breast milk apelin level increases with maternal obesity and high-fat feeding during lactation. Int J Obes 45, 1052–1060 (2021). https://doi.org/10.1038/s41366-021-00772-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41366-021-00772-y
This article is cited by
-
Programming of metabolism by adipokines during development
Nature Reviews Endocrinology (2023)