Abstract
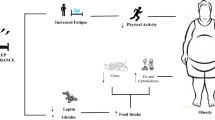
The interest on a potential association between cancer and sleep-disordered breathing (SDB) has clearly gained substantial traction over the last several years. This novel relationship was initially explored in experimental models of obstructive sleep apnea (OSA) and showed that both intermittent hypoxia and sleep fragmentation, the two main hallmarks of OSA, promoted alterations in both tumorigenesis and tumor malignant properties. In parallel, an intriguing role of obesity as a major interactive player in the relationship between cancer and OSA was postulated in the following contextual settings: (1) obesity (with or without OSA) is associated with increased risk of some types of cancer (both incidence and aggressiveness), whereas obesity could be protective for others (“obesity paradox”); (2) OSA has been associated with increased risk for some types of cancer (independent of obesity), but not with others; (3) More than 80% of adult patients with OSA are overweight and >50% are obese; (4) both OSA and obesity exhibit oscillations in tissue oxygen tensions in peripheral organs such as adipose tissues. Further understanding these complex relationships become all the more important considering that the prevalence of obesity, cancer and OSA are all increasing worldwide. In parallel, experimental models of OSA provide biological plausibility constructs to the clinical and epidemiological findings, suggesting that the metabolic and inflammatory changes induced by chronic intermittent hypoxia and sleep fragmentation may foster or exacerbate immune and biomechanical alterations of the tumor microenvironment, including the expression of extracellular matrix components facilitating tumor progression.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Physical status: the use and interpretation of anthropometry. Report of a WHO expert committee. World Health Organ Tech Rep Ser. 1995;854:1–452.
Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of US adults. N Engl J Med. 2003;348:1625–38.
Trinchieri G. Cancer and inflammation: an old intuition with rapidly evolving new concepts. Annu Rev Immunol. 2012;30:677–706.
Vainio H, Bianchini F. Prevent-weight control and physical activity. In: IARC handbooks of cancer 2019, vol. 6. Lyon: IARC Press; 2002.
Renehan AG, Tyson M, Egger M, Heller RF, Zwahlen M. Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet. 2008;371:569–78.
Lee DH, Giovannucci EL. The obesity paradox in cancer: epidemiologic insights and perspectives. Curr Nutr Rep. 2019;8:175–81.
Han SJ, Boyko EJ. The evidence for an obesity paradox in Type 2 diabetes mellitus. Diabetes Metab J. 2018;42:179–87.
Naderi N, Kleine CE, Park C, Hsiung JT, Soohoo M, Tantisattamo E, et al. Obesity paradox in advanced kidney disease: from bedside to the bench. Prog Cardiovasc Dis. 2018;61:168–81.
Elagizi A, Kachur S, Lavie CJ, Carbone S, Pandey A, Ortega FB, et al. An overview and update on obesity and the obesity paradox in cardiovascular diseases. Prog Cardiovasc Dis. 2018;61:142–50.
Lennon H, Sperrin M, Badrick E, Renehan AG. The obesity paradox in cancer: a review. Curr Oncol Rep. 2016;18:56.
Iyengar NM, Gucalp A, Dannenberg AJ, Hudis CA. Obesity and cancer mechanisms: tumor microenvironment and inflammation. J Clin Oncol. 2016;34:4270–6.
Deng T, Lyon CJ, Bergin S, Caligiuri MA, Hsueh WA. Obesity, inflammation, and cancer. Annu Rev Pathol. 2016;11:421–49.
Himbert C, Delphan M, Scherer D, Bowers LW, Hursting S, Ulrich CM. Signals from the adipose microenvironment and the obesity-cancer link—a systematic review. Cancer Prev Res. 2017;10:494–506.
Amin MN, Hussain MS, Sarwar MS, Rahman Moghal MM, Das A, Hossain MZ, et al. How the association between obesity and inflammation may lead to insulin resistance and cancer. Diabetes Metab Syndr. 2019;13:1213–24.
Ortega LS, Bradbury KE, Cross AJ, Morris JS, Gunter MJ, Murphy N. A Prospective investigation of body size, body fat composition and colorectal cancer risk in the UK biobank. Sci Rep. 2017;7:17807.
Jung IS, Shin CM, Park SJ, Park YS, Yoon H, Jo HJ, et al. Association of visceral adiposity and insulin resistance with colorectal adenoma and colorectal cancer. Intest Res. 2019;17:404–12.
Donohoe CL, Doyle SL, Reynolds JV. Visceral adiposity, insulin resistance and cancer risk. Diabetol Metab Syndr. 2011;3:12.
Framnes SN, Arble DM. The bidirectional relationship between obstructive sleep apnea and metabolic disease. Front Endocrinology. 2018;9:440.
Carneiro G, Zanella MT. Obesity metabolic and hormonal disorders associated with obstructive sleep apnea and their impact on the risk of cardiovascular events. Metabolism. 2018;84:76–84.
Sutherland K, Almeida FR, de Chazal P, Cistulli PA. Prediction in obstructive sleep apnoea: diagnosis, comorbidity risk, and treatment outcomes. Expert Rev Respir Med. 2018;12:293–307.
Peppard PE, Hagen EW. The last 25 years of obstructive sleep apnea epidemiology-and the next 25? Am J Respir Crit Care Med. 2018;197:310–2.
Franklin KA, Lindberg E. Obstructive sleep apnea is a common disorder in the population—a review on the epidemiology of sleep apnea. J Thorac Dis. 2015;7:1311–22.
Ruiz-Ojeda FJ, Mendez-Gutierrez A, Aguilera CM, Plaza-Diaz J. Extracellular matrix remodeling of adipose tissue in obesity and metabolic diseases. Int J Mol Sci. 2019;20:E4888. https://doi.org/10.3390/ijms20194888.
Lempesis IG, van Meijel RLJ, Manolopoulos KN, Goossens GH. Oxygenation of adipose tissue: a human perspective. Acta Physiol. 2020;228:e13298. https://doi.org/10.1111/apha.13298.
Gaspar JM, Velloso LA. Hypoxia inducible factor as a central regulator of metabolism—implications for the development of obesity. Front Neurosci. 2018;12:813.
Engin A. Adipose tissue hypoxia in obesity and its impact on preadipocytes and macrophages: hypoxia hypothesis. Adv Exp Med Biol. 2017;960:305–26.
Jo J, Gavrilova O, Pack S, Jou W, Mullen S, Sumner AE, et al. Hypertrophy and/or hyperplasia: dynamics of adipose tissue growth. PLoS Comput Biol. 2009;5:e1000324.
Semenza GL, Agani F, Feldser D, Iyer N, Kotch L, Laughner E, et al. Hypoxia, HIF-1, and the pathophysiology of common human diseases. Adv Exp Med Biol. 2000;475:123–30.
Ye J. Emerging role of adipose tissue hypoxia in obesity and insulin resistance. Int J Obes. 2009;33:54–66.
Ryan S, Arnaud C, Fitzpatrick SF, Gaucher J, Tamisier R, Pepin JL. Adipose tissue as a key player in obstructive sleep apnoea. Eur Respir Rev. 2019;28:190006.
Gozal D, Gileles-Hillel A, Cortese R, Li Y, Almendros I, Qiao Z, et al. Visceral white adipose tissue after chronic intermittent and sustained hypoxia in mice. Am J Respir Cell Mol Biol. 2017;56:477–87.
Almendros I, Farre R, Planas AM, Torres M, Bonsignore MR, Navajas D, et al. Tissue oxygenation in brain, muscle, and fat in a rat model of sleep apnea: differential effect of obstructive apneas and intermittent hypoxia. Sleep. 2011;34:1127–33.
Gileles-Hillel A, Almendros I, Khalyfa A, Nigdelioglu R, Qiao Z, Hamanaka RB, et al. Prolonged exposures to intermittent hypoxia promote visceral white adipose tissue inflammation in a murine model of severe sleep apnea: effect of normoxic recovery. Sleep. 2017;40. https://doi.org/10.1093/sleep/zsw074.
Shah N, Roux F. The relationship of obesity and obstructive sleep apnea. Clin Chest Med. 2009;30:455–65.
Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med. 1993;328:1230–5.
Young T, Shahar E, Nieto FJ, Redline S, Newman AB, Gottlieb DJ, et al. Predictors of sleep-disordered breathing in community-dwelling adults: the Sleep Heart Health Study. Arch Intern Med. 2002;162:893–900.
Dixon JB, Schachter LM, O’Brien PE. Polysomnography before and after weight loss in obese patients with severe sleep apnea. Int J Obes. 2005;29:1048–54.
Johansson K, Neovius M, Lagerros YT, Harlid R, Rossner S, Granath F, et al. Effect of a very low energy diet on moderate and severe obstructive sleep apnoea in obese men: a randomised controlled trial. BMJ. 2009;339:b4609.
Peppard PE, Young T, Palta M, Dempsey J, Skatrud J. Longitudinal study of moderate weight change and sleep-disordered breathing. J Am Med Assoc. 2000;284:3015–21.
Drager LF, Brunoni AR, Jenner R, Lorenzi-Filho G, Bensenor IM, Lotufo PA. Effects of CPAP on body weight in patients with obstructive sleep apnoea: a meta-analysis of randomised trials. Thorax. 2015;70:258–64.
Kapur V, Strohl KP, Redline S, Iber C, O’Connor G, Nieto J. Underdiagnosis of sleep apnea syndrome in U.S. communities. Sleep Breath. 2002;6:49–54.
Heinzer R, Vat S, Marques-Vidal P, Marti-Soler H, Andries D, Tobback N, et al. Prevalence of sleep-disordered breathing in the general population: the HypnoLaus study. Lancet Respir Med. 2015;3:310–8.
Benjafield AV, Ayas NT, Eastwood PR, Heinzer R, Ip MSM, Morrell MJ, et al. Estimation of the global prevalence and burden of obstructive sleep apnoea: a literature-based analysis. Lancet Respir Med. 2019;7:687–98.
Dreher M, Kruger S, Schulze-Olden S, Keszei A, Storre JH, Woehrle H, et al. Sleep-disordered breathing in patients with newly diagnosed lung cancer. BMC Pulm Med. 2018;18:72.
Martinez-Garcia MA, Campos-Rodriguez F, Almendros I, Garcia-Rio F, Sanchez-de-la-Torre M, Farre R, et al. Cancer and sleep apnea: cutaneous melanoma as a case study. Am J Respir Crit Care Med. 2019;200:1345–53.
Martinez-Garcia MA, Campos-Rodriguez F, Nagore E, Martorell A, Rodriguez-Peralto JL, Riveiro-Falkenbach E, et al. Sleep-disordered breathing is independently associated with increased aggressiveness of cutaneous melanoma: a multicenter observational study in 443 patients. Chest. 2018;154:1348–58.
Campos-Rodriguez F, Martinez-Garcia MA, Martinez M, Duran-Cantolla J, Pena ML, Masdeu MJ, et al. Association between obstructive sleep apnea and cancer incidence in a large multicenter Spanish cohort. Am J Respir Crit Care Med. 2013;187:99–105.
Christensen AS, Clark A, Salo P, Nymann P, Lange P, Prescott E, et al. Symptoms of sleep disordered breathing and risk of cancer: a prospective cohort study. Sleep. 2013;36:1429–35.
Marshall NS, Wong KK, Cullen SR, Knuiman MW, Grunstein RR. Sleep apnea and 20-year follow-up for all-cause mortality, stroke, and cancer incidence and mortality in the Busselton Health Study cohort. J Clin Sleep Med. 2014;10:355–62.
Chen JC, Hwang JH. Sleep apnea increased incidence of primary central nervous system cancers: a nationwide cohort study. Sleep Med. 2014;15:749–54.
Kendzerska T, Leung RS, Hawker G, Tomlinson G, Gershon AS. Obstructive sleep apnea and the prevalence and incidence of cancer. CMAJ. 2014;186:985–92.
Chang WP, Liu ME, Chang WC, Yang AC, Ku YC, Pai JT, et al. Sleep apnea and the subsequent risk of breast cancer in women: a nationwide population-based cohort study. Sleep Med. 2014;15:1016–20.
Palamaner Subash SG, Kumar AA, Cheskin LJ, Pancholy SB. Association between sleep-disordered breathing, obstructive sleep apnea, and cancer incidence: a systematic review and meta-analysis. Sleep Med. 2015;16:1289–94.
Zhang XB, Peng LH, Lyu Z, Jiang XT, Du YP. Obstructive sleep apnoea and the incidence and mortality of cancer: a meta-analysis. Eur J Cancer Care. 2017;26. https://doi.org/10.1111/ecc.12427.
Chen C-Y, Hu J-M, Shen C-J, Chou Y-C, Tian Y-F, Chen Y-C, et al. Increased incidence of colorectal cancer with obstructive sleep apnea: a nationwide population-based cohort study. Sleep Med. 2019;66:15–20.
Fang HF, Miao NF, Chen CD, Sithole T, Chung MH. Risk of cancer in patients with insomnia, parasomnia, and obstructive sleep apnea: a nationwide nested case-control study. J Cancer. 2015;6:1140–7.
Lin CL, Liu TC, Wang YN, Chung CH, Chien WC. The association between sleep disorders and the risk of colorectal cancer in patients: a population-based nested case-control study. In Vivo. 2019;33:573–9.
Nieto FJ, Peppard PE, Young T, Finn L, Hla KM, Farre R. Sleep-disordered breathing and cancer mortality: results from the Wisconsin Sleep Cohort Study. Am J Respir Crit Care Med. 2012;186:190–4.
Martinez-Garcia MA, Campos-Rodriguez F, Duran-Cantolla J, de la Pena M, Masdeu MJ, Gonzalez M, et al. Obstructive sleep apnea is associated with cancer mortality in younger patients. Sleep Med. 2014;15:742–8.
Martinez-Garcia MA, Martorell-Calatayud A, Nagore E, Valero I, Selma MJ, Chiner E, et al. Association between sleep disordered breathing and aggressiveness markers of malignant cutaneous melanoma. Eur Respir J. 2014;43:1661–8.
Gozal D, Ham SA, Mokhlesi B. Sleep apnea and cancer: analysis of a nationwide population sample. Sleep. 2016;39:1493–500.
Sillah A, Watson NF, Gozal D, Phipps AI. Obstructive sleep apnea severity and subsequent risk for cancer incidence. Prev Med Rep. 2019;15:100886.
Campos-Rodriguez F, Cruz-Medina A, Selma MJ, Rodriguez-de-la-Borbolla-Artacho, Sanchez-Vega A, Ripoll-Orts F, et al. Association between sleep-disordered breathing and breast cancer aggressiveness. PLoS ONE. 2018;13:e0207591.
Lee S, Kim BG, Kim JW, Lee KL, Koo DL, Nam H, et al. Obstructive sleep apnea is associated with an increased risk of colorectal neoplasia. Gastrointest Endosc. 2017;85:568–73.
Torres M, Campillo N, Nonaka PN, Montserrat JM, Gozal D, Martinez-Garcia MA, et al. Aging reduces intermittent hypoxia-induced lung carcinoma growth in a mouse model of sleep apnea. Am J Respir Crit Care Med. 2018;198:1234–6.
Virchow R. Cellular pathology. As based upon physiological and pathological histology. Lecture XVI-Atheromatous affection of arteries. 1858. Nutr Rev. 1989;47:23–5.
Gonzalez H, Hagerling C, Werb Z. Roles of the immune system in cancer: from tumor initiation to metastatic progression. Genes Dev. 2018;32:1267–84.
Palucka AK, Coussens LM. The basis of oncoimmunology. Cell. 2016;164:1233–47.
Farhood B, Najafi M, Mortezaee K. CD8(+) cytotoxic T lymphocytes in cancer immunotherapy: a review. J Cell Physiol. 2019;234:8509–21.
Almendros I, Wang Y, Becker L, Lennon FE, Zheng J, Coats BR, et al. Intermittent hypoxia-induced changes in tumor-associated macrophages and tumor malignancy in a mouse model of sleep apnea. Am J Respir Crit Care Med. 2014;189:593–601.
Gaines J, Vgontzas AN, Fernandez-Mendoza J, Bixler EO. Obstructive sleep apnea and the metabolic syndrome: the road to clinically-meaningful phenotyping, improved prognosis, and personalized treatment. Sleep Med Rev. 2018;42:211–9.
Carreras A, Zhang SX, Peris E, Qiao Z, Wang Y, Almendros I, et al. Effect of resveratrol on visceral white adipose tissue inflammation and insulin sensitivity in a mouse model of sleep apnea. Int J Obes. 2015;39:418–23.
Zhang SX, Khalyfa A, Wang Y, Carreras A, Hakim F, Neel BA, et al. Sleep fragmentation promotes NADPH oxidase 2-mediated adipose tissue inflammation leading to insulin resistance in mice. Int J Obes. 2014;38:619–24.
Poroyko VA, Carreras A, Khalyfa A, Khalyfa AA, Leone V, Peris E, et al. Chronic sleep disruption alters gut microbiota, induces systemic and adipose tissue inflammation and insulin resistance in mice. Sci Rep. 2016;6:35405.
Carreras A, Zhang SX, Almendros I, Wang Y, Peris E, Qiao Z, et al. Resveratrol attenuates intermittent hypoxia-induced macrophage migration to visceral white adipose tissue and insulin resistance in male mice. Endocrinology. 2015;156:437–43.
Moreno-Indias I, Torres M, Montserrat JM, Sanchez-Alcoholado L, Cardona F, Tinahones FJ, et al. Intermittent hypoxia alters gut microbiota diversity in a mouse model of sleep apnoea. Eur Respir J. 2015;45:1055–65.
Hakim F, Wang Y, Zhang SX, Zheng J, Yolcu ES, Carreras A, et al. Fragmented sleep accelerates tumor growth and progression through recruitment of tumor-associated macrophages and TLR4 signaling. Cancer Res. 2014;74:1329–37.
Almendros I, Gileles-Hillel A, Khalyfa A, Wang Y, Zhang SX, Carreras A, et al. Adipose tissue macrophage polarization by intermittent hypoxia in a mouse model of OSA: effect of tumor microenvironment. Cancer Lett. 2015;361:233–9.
Tiemessen MM, Jagger AL, Evans HG, van Herwijnen MJ, John S, Taams LS. CD4+CD25+Foxp3+ regulatory T cells induce alternative activation of human monocytes/macrophages. Proc Natl Acad Sci USA. 2007;104:19446–51.
Gallego-Martin T, Farre R, Almendros I, Gonzalez-Obeso E, Obeso A. Chronic intermittent hypoxia mimicking sleep apnoea increases spontaneous tumorigenesis in mice. Eur Respir J. 2017;49:1602111.
Cubillos-Zapata C, Avendano-Ortiz J, Hernandez-Jimenez E, Toledano V, Casas-Martin J, Varela-Serrano A, et al. Hypoxia-induced PD-L1/PD-1 crosstalk impairs T-cell function in sleep apnoea. Eur Respir J. 2017;50:1700833.
Akbarpour M, Khalyfa A, Qiao Z, Gileles-Hillel A, Almendros I, Farre R, et al. Altered CD8+ T-Cell lymphocyte function and TC1 cell stemness contribute to enhanced malignant tumor properties in murine models of sleep apnea. Sleep. 2017;40. https://doi.org/10.1093/sleep/zsw040.
Gaoatswe G, Kent BD, Corrigan MA, Nolan G, Hogan AE, McNicholas WT, et al. Invariant natural killer T cell deficiency and functional impairment in sleep apnea: links to cancer comorbidity. Sleep. 2015;38:1629–34.
Gharib SA, Seiger AN, Hayes AL, Mehra R, Patel SR. Treatment of obstructive sleep apnea alters transcriptional signatures in circulating leukocytes. Sleep. 2013;37:709–14.
White FM, Gatenby RA, Fischbach C. The physics of cancer. Cancer Res. 2019;79:2107–10.
Lu YC, Chu T, Hall MS, Fu DJ, Shi Q, Chiu A, et al. Physical confinement induces malignant transformation in mammary epithelial cells. Biomaterials. 2019;217:119307.
DelNero P, Hopkins BD, Cantley LC, Fischbach C. Cancer metabolism gets physical. Sci Transl Med. 2018;10. https://doi.org/10.1126/scitranslmed.aaq1011.
Northey JJ, Przybyla L, Weaver VM. Tissue force programs cell fate and tumor aggression. Cancer Discov. 2017;7:1224–37.
Roy CA, Gupta S, Chaturvedi PK, Kumar N, Pandey D. Mechanobiology of cancer stem cells and their niche. Cancer Microenviron. 2019;12:17–27.
Poltavets V, Kochetkova M, Pitson SM, Samuel MS. The role of the extracellular matrix and its molecular and cellular regulators in cancer cell plasticity. Front Oncol. 2018;8:431.
Rennhack JP, To B, Swiatnicki M, Dulak C, Ogrodzinski MP, Zhang Y, et al. Integrated analyses of murine breast cancer models reveal critical parallels with human disease. Nat Commun. 2019;10:3261.
Broders-Bondon F, Nguyen Ho-Bouldoires TH, Fernandez-Sanchez ME, Farge E. Mechanotransduction in tumor progression: the dark side of the force. J Cell Biol. 2018;217:1571–87.
Elosegui-Artola A, Andreu I, Beedle AEM, Lezamiz A, Uroz M, Kosmalska AJ, et al. Force triggers YAP nuclear entry by regulating transport across nuclear pores. Cell. 2017;171:1397–410.
Isermann P, Lammerding J. Nuclear mechanics and mechanotransduction in health and disease. Curr Biol. 2013;23:R1113–21.
Oudin MJ, Weaver VM. Physical and chemical gradients in the tumor microenvironment regulate tumor cell invasion, migration, and metastasis. Cold Spring Harb Symp Quant Biol. 2016;81:189–205.
Kaushik N, Kim S, Suh Y, Lee SJ. Proinvasive extracellular matrix remodeling for tumor progression. Arch Pharm Res. 2019;42:40–7.
Liu L, Zhang SX, Liao W, Farhoodi HP, Wong CW, Chen CC, et al. Mechanoresponsive stem cells to target cancer metastases through biophysical cues. Sci Transl Med. 2017;9:eaan2966.
Chandler EM, Saunders MP, Yoon CJ, Gourdon D, Fischbach C. Adipose progenitor cells increase fibronectin matrix strain and unfolding in breast tumors. Phys Biol. 2011;8:015008.
Gilkes DM, Semenza GL, Wirtz D. Hypoxia and the extracellular matrix: drivers of tumour metastasis. Nat Rev Cancer. 2014;14:430–9.
Ju JA, Godet I, Ye IC, Byun J, Jayatilaka H, Lee SJ, et al. Hypoxia selectively enhances integrin alpha5beta1 receptor expression in breast cancer to promote metastasis. Mol Cancer Res. 2017;15:723–34.
Petrova V, Annicchiarico-Petruzzelli M, Melino G, Amelio I. The hypoxic tumour microenvironment. Oncogenesis. 2018;7:10.
Rausch LK, Netzer NC, Hoegel J, Pramsohler S. The linkage between breast cancer, hypoxia, and adipose tissue. Front Oncol. 2017;7:211.
Gilkes DM, Bajpai S, Wong CC, Chaturvedi P, Hubbi ME, Wirtz D, et al. Procollagen lysyl hydroxylase 2 is essential for hypoxia-induced breast cancer metastasis. Mol Cancer Res. 2013;11:456–66.
He JY, Wei XH, Li SJ, Liu Y, Hu HL, Li ZZ, et al. Adipocyte-derived IL-6 and leptin promote breast cancer metastasis via upregulation of Lysyl Hydroxylase-2 expression. Cell Commun Signal. 2018;16:100.
Wang TH, Hsia SM, Shieh TM. Lysyl oxidase and the tumor microenvironment. Int J Mol Sci. 2016;18:E62. https://doi.org/10.3390/ijms18010062.
Almendros I, Montserrat JM, Torres M, Dalmases M, Cabanas ML, Campos-Rodriguez F, et al. Intermittent hypoxia increases melanoma metastasis to the lung in a mouse model of sleep apnea. Respir Physiol Neurobiol. 2013;186:303–7.
Marhuenda E, Campillo N, Gabasa M, Martinez-Garcia MA, Campos-Rodriguez F, Gozal D, et al. Effects of sustained and intermittent hypoxia on human lung cancer cells. Am J Respir Cell Mol Biol. 2019;61:540–4.
Farre N, Otero J, Falcones B, Torres M, Jorba I, Gozal D, et al. Intermittent hypoxia mimicking sleep apnea increases passive stiffness of myocardial extracellular matrix. a multiscale study. Front Physiol. 2018;9:1143.
Mesarwi OA, Shin MK, Drager LF, Bevans-Fonti S, Jun JC, Putcha N, et al. Lysyl oxidase as a serum biomarker of liver fibrosis in patients with severe obesity and obstructive sleep apnea. Sleep. 2015;38:1583–91.
Xu CY, Li DJ, Wu CL, Lou HJ, Jiang HW, Ding GQ. Serum sLOX-1 levels are correlated with the presence and severity of obstructive sleep apnea. Genet Test Mol Biomarkers. 2015;19:272–6.
Cozzo AJ, Fuller AM, Makowski L. Contribution of adipose tissue to development of cancer. Compr Physiol. 2017;8:237–82.
Lin ChunTH, Kang L. Adipose extracellular matrix remodelling in obesity and insulin resistance. Biochem Pharmacol. 2016;119:8–16.
Lengyel E, Makowski L, DiGiovanni J, Kolonin MG. Cancer as a matter of fat: the crosstalk between adipose tissue and tumors. Trends Cancer. 2018;4:374–84.
Quail DF, Dannenberg AJ. The obese adipose tissue microenvironment in cancer development and progression. Nat Rev Endocrinol. 2019;15:139–54.
Druso JE, Fischbach C. Biophysical properties of extracellular matrix: linking obesity and cancer. Trends Cancer. 2018;4:271–3.
Thorn CE, Knight B, Pastel E, McCulloch LJ, Patel B, Shore AC, et al. Adipose tissue is influenced by hypoxia of obstructive sleep apnea syndrome independent of obesity. Diabetes Metab. 2017;43:240–7.
Seo BR, Bhardwaj P, Choi S, Gonzalez J, Andresen Eguiluz RC, Wang K, et al. Obesity-dependent changes in interstitial ECM mechanics promote breast tumorigenesis. Sci Transl Med. 2015;7:301ra130.
Springer NL, Iyengar NM, Bareja R, Verma A, Jochelson M, Giri DD, et al. Obesity-associated extracellular matrix remodeling promotes a macrophage phenotype similar to tumor-associated macrophages. Am J Pathol. 2019;189:2019–35.
Chandler EM, Seo BR, Califano JP, Andresen Eguiluz RC, Lee JS, Yoon CJ, et al. Implanted adipose progenitor cells as physicochemical regulators of breast cancer. Proc Natl Acad Sci USA. 2012;109:9786–91.
Orecchioni S, Gregato G, Martin-Padura I, Reggiani F, Braidotti P, Mancuso P, et al. Complementary populations of human adipose CD34+ progenitor cells promote growth, angiogenesis, and metastasis of breast cancer. Cancer Res. 2013;73:5880–91.
Farre R, Almendros I, Montserrat JM, Gozal D, Navajas D. Gas partial pressure in cultured cells: patho-physiological importance and methodological approaches. Front Physiol. 2018;9:1803.
Broutier L, Mastrogiovanni G, Verstegen MM, Francies HE, Gavarro LM, Bradshaw CR, et al. Human primary liver cancer-derived organoid cultures for disease modeling and drug screening. Nat Med. 2017;23:1424–35.
Getova VE, van Dongen JA, Brouwer LA, Harmsen MC. Adipose tissue-derived ECM hydrogels and their use as 3D culture scaffold. Artif Cells Nanomed Biotechnol. 2019;47:1693–701.
Kumar V, Varghese S. Ex vivo tumor-on-a-chip platforms to study intercellular interactions within the tumor microenvironment. Adv Healthc Mater. 2019;8:e1801198.
Kingsley DM, Roberge CL, Rudkouskaya A, Faulkner DE, Barroso M, Intes X, et al. Laser-based 3D bioprinting for spatial and size control of tumor spheroids and embryoid bodies. Acta Biomater. 2019;95:357–70.
Ray A, Morford RK, Ghaderi N, Odde DJ, Provenzano PP. Dynamics of 3D carcinoma cell invasion into aligned collagen. Integr Biol. 2018;10:100–12.
Jorba I, Uriarte JJ, Campillo N, Farre R, Navajas D. Probing micromechanical properties of the extracellular matrix of soft tissues by atomic force microscopy. J Cell Physiol. 2016;232:19–26.
Colin-York H, Javanmardi Y, Barbieri L, Li D, Korobchevskaya K, Guo Y, et al. Spatiotemporally super-resolved volumetric traction force microscopy. Nano Lett. 2019;19:4427–34.
Donnelly D, Bajaj S, Yu J, Hsu M, Balar A, Pavlick A, et al. The complex relationship between body mass index and response to immune checkpoint inhibition in metastatic melanoma patients. J Immunother Cancer. 2019;7:222.
Funding
This work was supported in part by the Spanish Ministry of Economy and Competitiveness (SAF2017-85574-R) and Generalitat de Catalunya (Programa CERCA). IA is supported by SEPAR (595/2017) and DG by National Institutes of Health grants HL130984 and HL140548.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Almendros, I., Martinez-Garcia, M.A., Farré, R. et al. Obesity, sleep apnea, and cancer. Int J Obes 44, 1653–1667 (2020). https://doi.org/10.1038/s41366-020-0549-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41366-020-0549-z
This article is cited by
-
Dietary polyphenol intake, body composition and components of metabolic syndrome in a sample overweight and obese adults: a cross-sectional study
BMC Endocrine Disorders (2023)
-
Lower respiratory tract infections among newly diagnosed sleep apnea patients
BMC Pulmonary Medicine (2023)
-
A narrative review of the mechanisms and consequences of intermittent hypoxia and the role of advanced analytic techniques in pediatric autonomic disorders
Clinical Autonomic Research (2023)
-
Hybridization of soft-computing algorithms with neural network for prediction obstructive sleep apnea using biomedical sensor measurements
Neural Computing and Applications (2022)
-
Associating sleep problems with advanced cancer diagnosis, and immune checkpoint treatment outcomes: a pilot study
Supportive Care in Cancer (2022)