Abstract
Objective
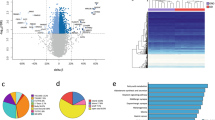
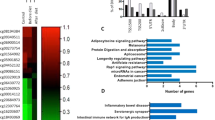
Both obesity and insulin resistance are characterized by severe long-term changes in the expression of many genes of importance in the regulation of metabolism. Because these changes occur throughout life, as a result of external factors, the disorders of gene expression could be epigenetically regulated.
Materials/methods
We analyzed the relationship between obesity and insulin resistance in enrolled patients by means of evaluation of the expression rate of numerous genes involved in the regulation of adipocyte metabolism and energy homeostasis in subcutaneous and visceral adipose tissue depots. We also investigated global and site-specific DNA methylation as one of the main regulators of gene expression. Visceral and subcutaneous adipose tissue biopsies were collected from 45 patients during abdominal surgery in an age range of 40–60 years.
Results
We demonstrated hypermethylation of PPARG, INSR, SLC2A4, and ADIPOQ promoters in obese patients with insulin resistance. Moreover, the methylation rate showed a negative correlation with the expression of the investigated genes. More, we showed a correlation between the expression of PPARG and the expression of numerous genes important for proper insulin action. Given the impact of PPARγ on the regulation of the cell insulin sensitivity through modulation of insulin pathway genes expression, hypermethylation in the PPARG promoter region may constitute one of the epigenetic pathways in the development of insulin resistance in obesity.
Conclusions
Our research shows that epigenetic regulation through excessive methylation may constitute a link between obesity and subsequent insulin resistance.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Aung K, Lorenzo C, Hinojosa MA, Haffner SM. Risk of developing diabetes and cardiovascular disease in metabolically unhealthy normal-weight and metabolically healthy obese individuals. J Clin Endocrinol Metab. 2014;99:462–8.
Nguyen DM, El-Serag HB. The epidemiology of obesity. Gastroenterol Clin N Am. 2010;39:1.
Smith CJ, Ryckman KK. Epigenetic and developmental influences on the risk of obesity, diabetes, and metabolic syndrome. Diabetes Metab Syndr Obes. 2015;8:295–302.
Berger SL, Kouzarides T, Shiekhattar R, Shilatifard A. An operational definition of epigenetics. Genes Dev. 2009;23:781.
Maor GL, Yearim A, Ast G. The alternative role of DNA methylation in splicing regulation. Trends Genet. 2015;31:274–80.
Zheng LD, Linarelli LE, Liu L, Wall SS, Greenawald MH, Seidel RW, et al. Insulin resistance is associated with epigenetic and genetic regulation of mitochondrial DNA in obese humans. Clin Epigenetics. 2015;7:60.
Arner P, Sinha I, Thorell A, Rydén M, Dahlman-Wright K, Dahlman I. The epigenetic signature of subcutaneous fat cells is linked to altered expression of genes implicated in lipid metabolism in obese women. Clin Epigenetics. 2015;7:93.
Pietiläinen KH, Ismail K, Järvinen E, Heinonen S, Tummers M, Bollepalli S, et al. DNA methylation and gene expression patterns in adipose tissue differ significantly within young adult monozygotic BMI-discordant twin pairs. Int J Obes. 2016;40:654–61.
Barberio MD, Nadler EP, Sevilla S, Lu R, Harmon B, Hubal MJ. Comparison of visceral adipose tissue DNA methylation and gene expression profiles in female adolescents with obesity. Diabetol Metab Syndr. 2019;11:98.
Simar D, Versteyhe S, Donkin I, Liu J, Hesson L, Nylander V, et al. DNA methylation is altered in B and NK lymphocytes in obese and type 2 diabetic human. Metabolism. 2014;63:1188–97.
Drogan D, Boeing H, Janke J, Schmitt B, Zhou Y, Walter J, et al. Regional distribution of body fat in relation to DNA methylation within the LPL, ADIPOQ and PPARγ promoters in subcutaneous adipose tissue. Nutr Diabetes. 2015;5:168.
Zhang Q, Xiao X, Zheng J, Li M, Yu M, Ping F, et al. A maternal high-fat diet induces DNA methylation changes that contribute to glucose intolerance in offspring. Front Endocrinol. 2019;10:871.
Choi K, Kim Y-B. Molecular mechanism of insulin resistance in obesity and type 2 diabetes. Korean J Intern Med. 2010;25:119–29.
Cash HL, McGarvey ST, Houseman EA, Marsit CJ, Hawley NL, Lambert-Messerlian GM, et al. Cardiovascular disease risk factors and DNA methylation at the LINE-1 repeat region in peripheral blood from Samoan Islanders. Epigenetics. 2011;6:1257–64.
Kim M, Long TI, Arakawa K, Wang R, Yu MC, Laird PW. DNA methylation as a biomarker for cardiovascular disease risk. PLoS ONE. 2010;5:3.
Medina-Gomez G, Gray S, Vidal-Puig A. Adipogenesis and lipotoxicity: role of peroxisome proliferator-activated receptor γ (PPARγ) and PPARγcoactivator-1 (PGC1). Public Health Nutr. 2007;10:1132–7.
Lehrke M, Lazar MA. The many faces of PPARγ. Cell. 2005;123:993–9.
Elstner E, Müller C, Koshizuka K, Williamson EA, Park D, Asou H, et al. Ligands for peroxisome proliferator-activated receptorγ and retinoic acid receptor inhibit growth and induce apoptosis of human breast cancer cells in vitro and in BNX mice. PNAS. 1998;95:8806–11.
Deeg MA, Tan MH. Pioglitazone versus Rosiglitazone: effects on lipids, lipoproteins, and apolipoproteins in head-to-head randomized clinical studies. PPAR Res. 2008;2008:520465.
He W, Barak Y, Hevener A, Olson P, Liao D, Le J, et al. Adipose-specific peroxisome proliferator-activated receptor γ knockout causes insulin resistance in fat and liver but not in muscle. PNAS. 2003;100:15712–7.
Koutnikova H, Cock T-A, Watanabe M, Houten SM, Champy M-F, Dierich A, et al. Compensation by the muscle limits the metabolic consequences of lipodystrophy in PPARγ hypomorphic mice. PNAS. 2003;100:14457–62.
Gustafson B, Jack MM, Cushman SW, Smith U. Adiponectin gene activation by thiazolidinediones requires PPARγ2, but not C/EBPα—evidence for differential regulation of the aP2 and adiponectin genes. Biochem Biophys Res Commun. 2003;308:933–9.
Fischer S, Navarrete Santos A, Thieme R, Ramin N, Fischer B. Adiponectin stimulates glucose uptake in rabbit blastocysts. Biol Reprod. 2010;83:859–65.
Berendoncks AMV, Stensvold D, Garnier A, Fortin D, Sente T, Vrints CJ, et al. Disturbed adiponectin—AMPK system in skeletal muscle of patients with metabolic syndrome. Eur J Prev Cardiolog. 2015;22:203–5.
Schindler M, Pendzialek M, Grybel KJ, Seeling T, Gürke J, Fischer B, et al. Adiponectin stimulates lipid metabolism via AMPK in rabbit blastocysts. Hum Reprod. 2017;32:1382.
Fasshauer M, Klein J, Neumann S, Eszlinger M, Paschke R. Hormonal regulation of adiponectin gene expression in 3T3-L1 adipocytes. Biochem Biophys Res Commun. 2002;290:1084–9.
Kern PA, Di Gregorio GB, Lu T, Rassouli N, Ranganathan G. Adiponectin expression from human adipose tissue: relation to obesity, insulin resistance, and tumor necrosis factor-alpha expression. Diabetes. 2003;52:1779–85.
Weyer C, Funahashi T, Tanaka S, Hotta K, Matsuzawa Y, Pratley RE, et al. Hypoadiponectinemia in obesity and type 2 diabetes: close association with insulin resistance and hyperinsulinemia. J Clin Endocrinol Metab. 2001;86:1930–5.
Hu E, Liang P, Spiegelman BM. AdipoQ is a novel adipose-specific gene dysregulated in obesity. J Biol Chem. 1996;271:10697–703.
Kim AY, Park YJ, Pan X, Shin KC, Kwak S-H, Bassas AF. et al. Obesity-induced DNA hypermethylation of the adiponectin gene mediates insulin resistance. Nat Commun. 2015;6:7585.
Cho YM, Youn B-S, Lee H, Lee N, Min S-S, Kwak SH, et al. Plasma retinol-binding protein-4 concentrations are elevated in human subjects with impaired glucose tolerance and type 2 diabetes. Diabetes Care. 2006;29:2457–61.
Mohapatra J, Sharma M, Acharya A, Pandya G, Chatterjee A, Balaraman R, et al. Retinol-binding protein 4: a possible role in cardiovascular complications. Br J Pharmacol. 2011;164:1939.
Öst A, Danielsson A, Lidén M, Eriksson U, Nystrom FH, Strålfors P. Retinol-binding protein-4 attenuates insulin-induced phosphorylation of IRS1 and ERK1/2 in primary human adipocytes. FASEB J. 2007;21:3696–704.
Acknowledgements
The project was funded by The National Science Centre, Poland. The number of the research project: 2016/21/D/NZ5/00155.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Cierzniak, A., Pawelka, D., Kaliszewski, K. et al. DNA methylation in adipocytes from visceral and subcutaneous adipose tissue influences insulin-signaling gene expression in obese individuals. Int J Obes 45, 650–658 (2021). https://doi.org/10.1038/s41366-020-00729-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41366-020-00729-7
This article is cited by
-
Trends in insulin resistance: insights into mechanisms and therapeutic strategy
Signal Transduction and Targeted Therapy (2022)
-
Prevalence of obesity and diabetes in older people with sarcopenia defined according to EWGSOP2 and FNHI criteria
Aging Clinical and Experimental Research (2022)