Abstract
Introduction
The hypothalamo-pituitary-adrenal (HPA) axis is perturbed in obesity. We previously reported presence of leptin resistance in the brainstem and uncoupling between central noradrenergic tone and the HPA axis in obesity-prone (DIO) rats. Metformin is shown to lower body weight and adiposity, but the underlying mechanism is unclear. We hypothesized that this is associated with restored HPA axis function.
Methods
Adult male DIO rats were placed on either a regular chow or HF diet for 7 weeks. Starting week 4, the animals were given either a low dose (60 mg/kg) or high dose (300 mg/kg) of metformin in drinking water. In addition to body weight and feeding, we examined different arms of the HPA axis to test if metformin can reinstate its function and coupling. To understand potential mechanisms, leptin signaling in the brainstem and circulating free fatty acid levels were also assessed.
Results
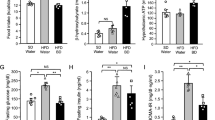
Metformin treatment lowered weight gain, fat mass, caloric intake, and serum leptin levels. HPA axis activity as determined by corticotropin-releasing hormone in the median eminence and serum corticosterone was decreased by metformin in a dose-dependent manner, and so was norepinephrine (NE) in the paraventricular nucleus. Importantly, metformin completely normalized the NE-HPA axis uncoupling. While brainstem pSTAT-3 and SOCS-3, key markers of leptin signaling, were not different between groups, circulating saturated and unsaturated free fatty acids were reduced in HF-fed, metformin-treated animals.
Conclusions
These findings suggest that oral metformin can successfully correct HPA axis dysfunction that is associated with lowered circulating free fatty acids in DIO rats, thereby uncovering a novel effect of metformin in the treatment of obesity.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Marin P, Darin N, Amemiya T, Andersson B, Jern S, Bjorntorp P. Cortisol secretion in relation to body fat distribution in obese premenopausal women. Metabolism. 1992;41:882–6.
Pasquali R, Cantobelli S, Casimirri F, Capelli M, Bortoluzzi L, Flamia R, et al. The hypothalamic-pituitary-adrenal axis in obese women with different patterns of body fat distribution. J Clin Endocrinol Metab. 1993;77:341–6.
Duclos M, Corcuff JB, Etcheverry N, Rashedi M, Tabarin A, Roger P. Abdominal obesity increases overnight cortisol excretion. J Endocrinol Investig. 1999;22:465–71.
Pasquali R, Biscotti D, Spinucci G, Vicennati V, Genazzani AD, Sgarbi L, et al. Pulsatile secretion of ACTH and cortisol in premenopausal women: effect of obesity and body fat distribution. Clin Endocrinol. 1998;48:603–12.
Levin BE, Richard D, Michel C, Servatius R. Differential stress responsivity in diet-induced obese and resistant rats. Am J Physiol Regul Integr Comp Physiol. 2000;279:R1357–64.
Nicolaides NC, Kyratzi E, Lamprokostopoulou A, Chrousos GP, Charmandari E. Stress, the stress system and the role of glucocorticoids. Neuroimmunomodulation. 2015;22:6–19.
Tsigos C, Chrousos GP. Hypothalamic-pituitary-adrenal axis, neuroendocrine factors and stress. J Psychosom Res. 2002;53:865–71.
Bose M, Olivan B, Laferrere B. Stress and obesity: the role of the hypothalamic-pituitary-adrenal axis in metabolic disease. Curr Opin Endocrinol Diabetes Obes. 2009;16:340–6.
Bugajski J, Turon M, Gadek-Michalska A, Borycz JA. Catecholaminergic regulation of the hypothalamic-pituitary-adrenocortical activity. J Physiol Pharmacol. 1991;42:93–103.
Guillaume V, Conte-Devolx B, Szafarczyk A, Malaval F, Pares-Herbute N, Grino M, et al. The corticotropin-releasing factor release in rat hypophysial portal blood is mediated by brain catecholamines. Neuroendocrinology. 1987;46:143–6.
Szafarczyk A, Malaval F, Laurent A, Gibaud R, Assenmacher I. Further evidence for a central stimulatory action of catecholamines on adrenocorticotropin release in the rat. Endocrinology. 1987;121:883–92.
Shin AC, MohanKumar SM, Sirivelu MP, Claycombe KJ, Haywood JR, Fink GD, et al. Chronic exposure to a high-fat diet affects stress axis function differentially in diet-induced obese and diet-resistant rats. Int J Obes. 2010;34:1218–26.
Shin AC, MohanKumar SMJ, Balasubramanian P, Sirivelu MP, Linning K, Woolcock A, et al. Responsiveness of hypothalamo-pituitary-adrenal axis to leptin is impaired in diet-induced obese rats. Nutr Diabetes. 2019;9:10.
DeFronzo RA, Goodman AM. Efficacy of metformin in patients with non-insulin-dependent diabetes mellitus. The Multicenter Metformin Study Group. N Engl J Med. 1995;333:541–9.
Rena G, Hardie DG, Pearson ER. The mechanisms of action of metformin. Diabetologia. 2017;60:1577–85.
Stumvoll M, Nurjhan N, Perriello G, Dailey G, Gerich JE. Metabolic effects of metformin in non-insulin-dependent diabetes mellitus. N Engl J Med. 1995;333:550–4.
Apolzan JW, Venditti EM, Edelstein SL, Knowler WC, Dabelea D, Boyko EJ, et al. Long-term weight loss with metformin or lifestyle intervention in the diabetes prevention program outcomes study. Ann Intern Med. 2019;170:682–90.
Lentferink YE, Knibbe CAJ, van der Vorst MMJ. Efficacy of metformin treatment with respect to weight reduction in children and adults with obesity: a systematic review. Drugs. 2018;78:1887–901.
Warnakulasuriya LS, Fernando MMA, Adikaram AVN, Thawfeek ARM, Anurasiri WL, Silva RR, et al. Metformin in the management of childhood obesity: a randomized control trial. Child Obes. 2018;14:553–65.
Rouru J, Huupponen R, Pesonen U, Koulu M. Subchronic treatment with metformin produces anorectic effect and reduces hyperinsulinemia in genetically obese Zucker rats. Life Sci. 1992;50:1813–20.
Duca FA, Cote CD, Rasmussen BA, Zadeh-Tahmasebi M, Rutter GA, Filippi BM, et al. Metformin activates a duodenal Ampk-dependent pathway to lower hepatic glucose production in rats. Nat Med. 2015;21:506–11.
Aubert G, Mansuy V, Voirol MJ, Pellerin L, Pralong FP. The anorexigenic effects of metformin involve increases in hypothalamic leptin receptor expression. Metabolism. 2011;60:327–34.
Labuzek K, Suchy D, Gabryel B, Bielecka A, Liber S, Okopien B. Quantification of metformin by the HPLC method in brain regions, cerebrospinal fluid and plasma of rats treated with lipopolysaccharide. Pharmacol Rep. 2010;62:956–65.
Lv WS, Wen JP, Li L, Sun RX, Wang J, Xian YX, et al. The effect of metformin on food intake and its potential role in hypothalamic regulation in obese diabetic rats. Brain Res. 2012;1444:11–9.
Derkach KV, Zakharova IO, Romanova IV, Zorina II, Mikhrina AL, Shpakov AO. Metabolic parameters and functional state of hypothalamic signaling systems in A(Y)/a mice with genetic predisposition to obesity and the effect of metformin. Dokl Biochem Biophys. 2017;477:377–81.
Kim YW, Kim JY, Park YH, Park SY, Won KC, Choi KH, et al. Metformin restores leptin sensitivity in high-fat-fed obese rats with leptin resistance. Diabetes. 2006;55:716–24.
Levin BE, Dunn-Meynell AA, Balkan B, Keesey RE. Selective breeding for diet-induced obesity and resistance in Sprague-Dawley rats. Am J Physiol. 1997;273:R725–30.
Rouru J, Pesonen U, Koulu M, Huupponen R, Santti E, Virtanen K, et al. Anorectic effect of metformin in obese Zucker rats: lack of evidence for the involvement of neuropeptide Y. Eur J Pharmacol. 1995;273:99–106.
Rouru J, Huupponen R, Santti E, Koulu M. Effect of subchronic metformin treatment on macronutrient selection in genetically obese Zucker rats. Pharmacol Toxicol. 1993;72:300–3.
Paxinos G. The rat brain in streotaxic coordinates, 2nd ed. Academic: San Diego, CA; 1987.
Francis J, MohanKumar SM, MohanKumar PS. Leptin inhibits norepinephrine efflux from the hypothalamus in vitro: role of gamma aminobutyric acid. Brain Res. 2004;1021:286–91.
Clark KA, MohanKumar SM, Kasturi BS, MohanKumar PS. Effects of central and systemic administration of leptin on neurotransmitter concentrations in specific areas of the hypothalamus. Am J Physiol Regul Integr Comp Physiol. 2006;290:R306–12.
Clark KA, Shin AC, Sirivelu MP, Mohankumar SM, Mohankumar PS. Systemic administration of leptin decreases plasma corticosterone levels: role of hypothalamic norepinephrine. Brain Res. 2008;1195:89–95.
Contreras GA, O’Boyle NJ, Herdt TH, Sordillo LM. Lipomobilization in periparturient dairy cows influences the composition of plasma nonesterified fatty acids and leukocyte phospholipid fatty acids. J Dairy Sci. 2010;93:2508–16.
Jequier E. Leptin signaling, adiposity, and energy balance. Ann N Y Acad Sci. 2002;967:379–88.
Freemark M, Bursey D. The effects of metformin on body mass index and glucose tolerance in obese adolescents with fasting hyperinsulinemia and a family history of type 2 diabetes. Pediatrics. 2001;107:E55.
Worsley R, Jane F, Robinson PJ, Bell RJ, Davis SR. Metformin for overweight women at midlife: a double-blind, randomized, controlled trial. Climacteric. 2015;18:270–7.
Pacak K, McCarty R, Palkovits M, Cizza G, Kopin IJ, Goldstein DS, et al. Decreased central and peripheral catecholaminergic activation in obese Zucker rats. Endocrinology. 1995;136:4360–7.
Berridge CW, Espana RA, Vittoz NM. Hypocretin/orexin in arousal and stress. Brain Res. 2010;1314:91–102.
Bonnavion P, Jackson AC, Carter ME, de Lecea L. Antagonistic interplay between hypocretin and leptin in the lateral hypothalamus regulates stress responses. Nat Commun. 2015;6:6266.
Heiman ML, Ahima RS, Craft LS, Schoner B, Stephens TW, Flier JS. Leptin inhibition of the hypothalamic-pituitary-adrenal axis in response to stress. Endocrinology. 1997;138:3859–63.
Hay-Schmidt A, Helboe L, Larsen PJ. Leptin receptor immunoreactivity is present in ascending serotonergic and catecholaminergic neurons of the rat. Neuroendocrinology. 2001;73:215–26.
Hosoi T, Kawagishi T, Okuma Y, Tanaka J, Nomura Y. Brain stem is a direct target for leptin’s action in the central nervous system. Endocrinology. 2002;143:3498–504.
Benthem L, Keizer K, Wiegman CH, de Boer SF, Strubbe JH, Steffens AB, et al. Excess portal venous long-chain fatty acids induce syndrome X via HPA axis and sympathetic activation. Am J Physiol Endocrinol Metab. 2000;279:E1286–93.
Widmaier EP, Margenthaler J, Sarel I. Regulation of pituitary-adrenocortical activity by free fatty acids in vivo and in vitro. Prostaglandins Leukot Essent Fatty Acids. 1995;52:179–83.
Widmaier EP, Rosen K, Abbott B. Free fatty acids activate the hypothalamic-pituitary-adrenocortical axis in rats. Endocrinology. 1992;131:2313–8.
Seifarth C, Schehler B, Schneider HJ. Effectiveness of metformin on weight loss in non-diabetic individuals with obesity. Exp Clin Endocrinol Diabetes. 2013;121:27–31.
Duan Y, Zhang R, Zhang M, Sun L, Dong S, Wang G, et al. Metformin inhibits food intake and neuropeptide Y gene expression in the hypothalamus. Neural Regen Res. 2013;8:2379–88.
Schommers P, Thurau A, Bultmann-Mellin I, Guschlbauer M, Klatt AR, Rozman J, et al. Metformin causes a futile intestinal-hepatic cycle which increases energy expenditure and slows down development of a type 2 diabetes-like state. Mol Metab. 2017;6:737–47.
Bauman V, Ariel-Donges AH, Gordon EL, Daniels MJ, Xu D, Ross KM, et al. Effect of dose of behavioral weight loss treatment on glycemic control in adults with prediabetes. BMJ Open Diabetes Res Care. 2019;7:e000653.
Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393–403.
Perry RJ, Resch JM, Douglass AM, Madara JC, Rabin-Court A, Kucukdereli H, et al. Leptin’s hunger-suppressing effects are mediated by the hypothalamic-pituitary-adrenocortical axis in rodents. Proc Natl Acad Sci USA. 2019;116:13670–79.
Perry RJ, Zhang XM, Zhang D, Kumashiro N, Camporez JP, Cline GW, et al. Leptin reverses diabetes by suppression of the hypothalamic-pituitary-adrenal axis. Nat Med. 2014;20:759–63.
Leibowitz SF. Paraventricular nucleus: a primary site mediating adrenergic stimulation of feeding and drinking. Pharmacol Biochem Behav. 1978;8:163–75.
Leibowitz SF, Brown O, Tretter JR, Kirschgessner A. Norepinephrine, clonidine, and tricyclic antidepressants selectively stimulate carbohydrate ingestion through noradrenergic system of the paraventricular nucleus. Pharmacol Biochem Behav. 1985;23:541–50.
Zhao S, Zhu Y, Schultz RD, Li N, He Z, Zhang Z, et al. Partial leptin reduction as an insulin sensitization and weight loss strategy. Cell Metab. 2019;30:706–19.e6.
Ibars M, Ardid-Ruiz A, Suarez M, Muguerza B, Blade C, Aragones G. Proanthocyanidins potentiate hypothalamic leptin/STAT3 signalling and Pomc gene expression in rats with diet-induced obesity. Int J Obes. 2017;41:129–36.
Sahu A. Intracellular leptin-signaling pathways in hypothalamic neurons: the emerging role of phosphatidylinositol-3 kinase-phosphodiesterase-3B-cAMP pathway. Neuroendocrinology. 2011;93:201–10.
Kullmann S, Kleinridders A, Small DM, Fritsche A, Haring HU, Preissl H, et al. Central nervous pathways of insulin action in the control of metabolism and food intake. Lancet Diabetes Endocrinol. 2020;8:524–34.
Kullmann S, Valenta V, Wagner R, Tschritter O, Machann J, Haring HU, et al. Brain insulin sensitivity is linked to adiposity and body fat distribution. Nat Commun. 2020;11:1841.
Cassaglia PA, Shi Z, Brooks VL. Insulin increases sympathetic nerve activity in part by suppression of tonic inhibitory neuropeptide Y inputs into the paraventricular nucleus in female rats. Am J Physiol Regul Integr Comp Physiol. 2016;311:R97–103.
Shi Z, Zhao D, Cassaglia PA, Brooks VL. Sites and sources of sympathoexcitation in obese male rats: role of brain insulin. Am J Physiol Regul Integr Comp Physiol. 2020;318:R634–48.
Stocker SD, Gordon KW. Glutamate receptors in the hypothalamic paraventricular nucleus contribute to insulin-induced sympathoexcitation. J Neurophysiol. 2015;113:1302–9.
Chan O, Inouye K, Akirav E, Park E, Riddell MC, Vranic M, et al. Insulin alone increases hypothalamo-pituitary-adrenal activity, and diabetes lowers peak stress responses. Endocrinology. 2005;146:1382–90.
Petersen JS, Liu W, Kapusta DR, Varner KJ. Metformin inhibits ganglionic neurotransmission in renal nerves. Hypertension. 1997;29:1173–7.
Manzella D, Grella R, Esposito K, Giugliano D, Barbagallo M, Paolisso G. Blood pressure and cardiac autonomic nervous system in obese type 2 diabetic patients: effect of metformin administration. Am J Hypertens. 2004;17:223–7.
Karise I, Bargut TC, Del Sol M, Aguila MB, Mandarim-de-Lacerda CA. Metformin enhances mitochondrial biogenesis and thermogenesis in brown adipocytes of mice. Biomed Pharmacother. 2019;111:1156–65.
Geerling JJ, Boon MR, van der Zon GC, van den Berg SA, van den Hoek AM, Lombes M, et al. Metformin lowers plasma triglycerides by promoting VLDL-triglyceride clearance by brown adipose tissue in mice. Diabetes. 2014;63:880–91.
Acknowledgements
We would like to thank Ms. Katrina Linning for her excellent technical assistance and for maintaining the rat colony.
Funding
This study was supported in part by NIH grant R01 AG027697 to PSM and NSF grant IBN0236385 to SMJM and PSM. ACS was supported by Biomedical Health Research Initiative grant, MSU.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Shin, A.C., Balasubramanian, P., Suryadevara, P. et al. Metformin effectively restores the HPA axis function in diet-induced obese rats. Int J Obes 45, 383–395 (2021). https://doi.org/10.1038/s41366-020-00688-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41366-020-00688-z