Abstract
Objective
Detailed data on adipokines and body composition during and after pregnancy in women of different BMI categories are lacking. Furthermore, adipokine regulation during pregnancy and the factors contributing to gestational insulin resistance are not completely understood. The objective was to longitudinally determine adipokine levels, body composition, and insulin sensitivity during and after pregnancy in women of healthy weight (HW) and with obesity (OB), and identify factors associated with insulin resistance.
Design
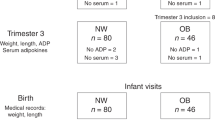
Women (30 HW, 19 OB) underwent blood sampling and body composition examination, by air-displacement plethysmography, longitudinally during pregnancy (trimesters 1, 2, 3) and after pregnancy (6, 12, 18 months postpartum). Serum leptin, soluble leptin receptor (sOB-R), and adiponectin levels were measured and free leptin index (FLI) and homeostatic model assessment of insulin resistance (HOMA-IR) determined.
Results
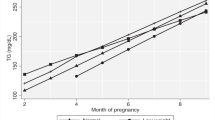
Fat mass and leptin increased during pregnancy in the HW (p < 0.01) but not in the OB group. sOB-R increased during pregnancy in both groups (p < 0.001). Thus, FLI was unchanged in HW throughout pregnancy but reduced in OB (p = 0.001), although consistently higher in OB. Adiponectin decreased in both groups during pregnancy (p < 0.001 for HW, p = 0.01 for OB). After pregnancy, adiponectin increased in both groups, but more markedly in OB where it reached trimester 1 levels. Multivariable regression identified FLI as the variable most strongly associated with HOMA-IR in all trimesters, but not after pregnancy.
Conclusions
Leptin, sOB-R, adiponectin, and FLI undergo marked changes during and after pregnancy with differences in women of different BMI. We suggest that leptin activity is regulated by its soluble receptor and that this is an important factor for optimizing fat mass and insulin sensitivity during pregnancy.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Linne Y. Effects of obesity on women’s reproduction and complications during pregnancy. Obesity Rev. 2004;5:137–43.
Cedergren M. Effects of gestational weight gain and body mass index on obstetric outcome in Sweden. Int J Gynaecol Obstet. 2006;93:269–74.
Gunderson E, Abrams B, Selvin S. Does the pattern of postpartum weight change differ according to pregravid body size? Int J Obes Relat Metab Disord. 2001;25:853–62.
Briana DD, Malamitsi-Puchner A. Reviews: adipocytokines in normal and complicated pregnancies. Reprod Sci. 2009;16:921–37.
Park H-K, Ahima RS. Physiology of leptin: energy homeostasis, neuroendocrine function and metabolism. Metabolism. 2015;64:24–34.
Tessier D, Ferraro Z, Gruslin A. Role of leptin in pregnancy: consequences of maternal obesity. Placenta. 2013;34:205–11.
Ladyman S, Augustine R, Grattan D. Hormone interactions regulating energy balance during pregnancy. J Neuroendocrinol. 2010;22:805–17.
Chan JL, Blüher S, Yiannakouris N, Suchard MA, Kratzsch J, Mantzoros CS. Regulation of circulating soluble leptin receptor levels by gender, adiposity, sex steroids, and leptin: observational and interventional studies in humans. Diabetes. 2002;51:2105–12.
Ingelsson E, Larson MG, Yin X, Wang TJ, Meigs JB, Lipinska I, et al. Circulating ghrelin, leptin, and soluble leptin receptor concentrations and cardiometabolic risk factors in a community-based sample. J Clin Endocrinol Metab. 2008;93:3149–57.
Page-Wilson G, Reitman-Ivashkov E, Meece K, White A, Rosenbaum M, Smiley R, et al. Cerebrospinal fluid levels of leptin, proopiomelanocortin, and agouti-related protein in human pregnancy: evidence for leptin resistance. J Clin Endocrinol Metab. 2013;98:264–71.
Andersson-Hall U, Svedin P, Andreasson U, Gren M, Ingemansson A, Zetterberg H, et al. Central and peripheral leptin and agouti-related protein during and after pregnancy in relation to weight change. Clin Endocrinol. 2018;88:263–71.
Nuamah MA, Sagawa N, Yura S, Mise H, Itoh H, Ogawa Y, et al. Free-to-total leptin ratio in maternal plasma is constant throughout human pregnancy. Endocr J. 2003;50:421–8.
Lewandowski K, Horn R, O’Callaghan C, Dunlop D, Medley G, O’Hare P, et al. Free leptin, bound leptin, and soluble leptin receptor in normal and diabetic pregnancies. J Clin Endocrinol Metab. 1999;84:300–6.
Sommer C, Gulseth HL, Jenum AK, Sletner L, Thorsby PM, Birkeland KI. Soluble leptin receptor and risk of gestational diabetes in a multiethnic population: a prospective cohort study. J Clin Endocrinol Metab. 2016;101:4070–5.
Hinkle SN, Rawal S, Liu D, Chen J, Tsai MY, Zhang C. Maternal adipokines longitudinally measured across pregnancy and their associations with neonatal size, length, and adiposity. Int J Obes. 2019;43:1422–34.
Berg AH, Combs TP, Scherer PE. ACRP30/adiponectin: an adipokine regulating glucose and lipid metabolism. Trends Endocrinol Metabolism. 2002;13:84–89.
Aye IL, Powell TL, Jansson T. Review: Adiponectin–the missing link between maternal adiposity, placental transport and fetal growth? Placenta. 2013;34:S40–S45.
Hendler I, Blackwell SC, Mehta SH, Whitty JE, Russell E, Sorokin Y, et al. The levels of leptin, adiponectin, and resistin in normal weight, overweight, and obese pregnant women with and without preeclampsia. Am J Obstet Gynecol 2005;193:979–83.
Lekva T, Roland MCP, Michelsen AE, Friis CM, Aukrust P, Bollerslev J, et al. Large reduction in adiponectin during pregnancy is associated with large-for-gestational-age newborns. J Clin Endocrinol Metab. 2017;102:2552–9.
Chen C, Xu X, Yan Y. Estimated global overweight and obesity burden in pregnant women based on panel data model. PLoS ONE. 2018;13:e0202183.
Bosaeus M, Hussain A, Karlsson T, Andersson L, Hulthen L, Svelander C, et al. A randomized longitudinal dietary intervention study during pregnancy: effects on fish intake, phospholipids, and body composition. Nutr J. 2015;14:1.
Svensson H, Wetterling L, Bosaeus M, Oden B, Oden A, Jennische E, et al. Body fat mass and the proportion of very large adipocytes in pregnant women are associated with gestational insulin resistance. Int J Obes. 2016;40:646–53.
Van Raaij J, Peek M, Vermaat-Miedema SH, Schonk CM, Hautvast J. New equations for estimating body fat mass in pregnancy from body density or total body water. Am J Clin Nutr. 1988;48:24–29.
Hopkinson JM, Butte NF, Ellis KJ, Wong WW, Puyau MR, Smith E. Body fat estimation in late pregnancy and early postpartum: comparison of two-, three-, and four-component models. Am J Clin Nutr. 1997;65:432–8.
National Research Council. Weight gain during pregnancy: reexamining the guidelines. Washington, DC: National Academies Press; 2010.
Kratzsch J, Lammert A, Bottner A, Seidel B, Mueller G, Thiery J, et al. Circulating soluble leptin receptor and free leptin index during childhood, puberty, and adolescence. J Clin Endocrinol Metab. 2002;87:4587–94.
Wallace TM, Levy JC, Matthews DR. Use and abuse of HOMA modeling. Diabetes Care. 2004;27:1487–95.
Schaab M, Kratzsch J. The soluble leptin receptor. Best Pract Res Clin Endocrinol Metab. 2015;29:661–70.
Tu H, Kastin AJ, Hsuchou H, Pan W. Soluble receptor inhibits leptin transport. J Cell Physiol. 2008;214:301–5.
van Dielen FM, van ‘t Veer C, Buurman WA, Greve JW. Leptin and soluble leptin receptor levels in obese and weight-losing individuals. J Clin Endocrinol Metab. 2002;87:1708–16.
Gavrilova O, Barr V, Marcus-Samuels B, Reitman M. Hyperleptinemia of pregnancy associated with the appearance of a circulating form of the leptin receptor. J Biol Chem. 1997;272:30546–51.
Farley D, Choi J, Dudley D, Li C, Jenkins S, Myatt L, et al. Placental amino acid transport and placental leptin resistance in pregnancies complicated by maternal obesity. Placenta. 2010;31:718–24.
Mørkrid K, Jenum AK, Sletner L, Vårdal MH, Waage CW, Nakstad B, et al. Failure to increase insulin secretory capacity during pregnancy-induced insulin resistance is associated with ethnicity and gestational diabetes. Eur J Endocrinol. 2012;167:579–88.
Vinter C, Jørgensen J, Ovesen P, Beck‐Nielsen H, Skytthe A, Jensen D. Metabolic effects of lifestyle intervention in obese pregnant women. Results from the randomized controlled trial ‘Lifestyle in Pregnancy’(LiP). Diabet Med. 2014;31:1323–30.
Sun Q, van Dam RM, Meigs JB, Franco OH, Mantzoros CS, Hu FB. Leptin and soluble leptin receptor levels in plasma and risk of type 2 diabetes in US women: a prospective study. Diabetes. 2010;59:611–8.
Sommer C, Lee S, Gulseth HL, Jensen J, Drevon CA, Birkeland KI. Soluble leptin receptor predicts insulin sensitivity and correlates with upregulation of metabolic pathways in men. J Clin Endocrinol Metab. 2017;103:1024–32.
Chen D, Xia G, Xu P, Dong M. Peripartum serum leptin and soluble leptin receptor levels in women with gestational diabetes. Acta Obst et Gynecol Scand. 2010;89:1595–9.
Krizova J, Eretova V, Haluzikova D, Anderlova K, Housova J, Kotrlikova E, et al. Soluble leptin receptor and leptin levels in pregnant women before and after delivery. Endocrine Res. 2004;30:379–85.
Misra VK, Trudeau S. The influence of overweight and obesity on longitudinal trends in maternal serum leptin levels during pregnancy. Obesity. 2011;19:416–21.
Butte N, Hopkinson J, Nicolson M. Leptin in human reproduction: serum leptin levels in pregnant and lactating women. J Clin Endocrinol Metab. 1997;82:585–9.
Masuzaki H, Ogawa Y, Sagawa N, Hosoda K, Matsumoto T, Mise H, et al. Nonadipose tissue production of leptin: leptin as a novel placenta derived hormone in humans. Nat Med. 1997;3:1029–33.
Sagawa N, Yura S, Itoh H, Kakui K, Takemura M, Nuamah MA, et al. Possible role of placental leptin in pregnancy. Endocrine. 2002;19:65–71.
Fuglsang J, Skjærbæk C, Frystyk J, Flyvbjerg A, Ovesen P. A longitudinal study of serum adiponectin during normal pregnancy. BJOG. 2006;113:110–3.
Henriksson P, Lof M, Forsum E. Parental fat-free mass is related to the fat-free mass of infants and maternal fat mass is related to the fat mass of infant girls. Acta Paediatr. 2015;104:491–7.
Rode L, Kjærgaard H, Ottesen B, Damm P, Hegaard HK. Association between gestational weight gain according to body mass index and postpartum weight in a large cohort of Danish women. Mat Child Health J. 2012;16:406–13.
Löf M, Hilakivi-Clarke L, Sandin S, Weiderpass E. Effects of pre-pregnancy physical activity and maternal BMI on gestational weight gain and birth weight. Acta Obst et Gynecol Scand. 2008;87:524–30.
Moll U, Olsson H, Landin-Olsson M. Impact of pregestational weight and weight gain during pregnancy on long-term risk for diseases. PLoS ONE. 2017;12:e0168543.
Ronnberg A, Hanson U, Ostlund I, Nilsson K. Effects on postpartum weight retention after antenatal lifestyle intervention–a secondary analysis of a randomized controlled trial. Acta Obst et Gynecol Scand. 2016;95:999–1007.
Acknowledgements
This work was supported by grants from the Emil and Wera Cornell Foundation, the Swedish Research Council (12206), the Swedish Diabetes Association Research Foundation (2015-08) and the Swedish state under the agreement between the Swedish government and the country councils, the ALF-agreement (720851). We would also like to thank all participating women of the PONCH study, and registered dietician Evelina Järvinen for expert caretaking and measurements during study visits.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Andersson-Hall, U., Svedin, P., Svensson, H. et al. Longitudinal changes in adipokines and free leptin index during and after pregnancy in women with obesity. Int J Obes 44, 675–683 (2020). https://doi.org/10.1038/s41366-019-0452-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41366-019-0452-7
This article is cited by
-
Maternal early mid-pregnancy adiponectin in relation to infant birth weight and the likelihood of being born large-for-gestational-age
Scientific Reports (2023)
-
Periconceptional biomarkers for maternal obesity: a systematic review
Reviews in Endocrine and Metabolic Disorders (2023)
-
Infant body composition relationship to maternal adipokines and fat mass: the PONCH study
Pediatric Research (2021)
-
Physical activity during pregnancy and association with changes in fat mass and adipokines in women of normal-weight or with obesity
Scientific Reports (2021)