Abstract
Background
Neuronal growth regulator 1 (NEGR1) is a glycosylphosphatidylinositol-anchored membrane protein that mediates neural cell communication and synapse formation. Multiple genome-wide association studies have reported that variations in NEGR1 are associated with human body weight control. Recently, we found that NEGR1 is involved in intracellular cholesterol trafficking, suggesting that it performs a non-central nervous system (CNS) function associated with human obesity.
Methods
We compared peripheral tissues such as the adipose, liver, and skeletal muscle tissues of Negr1−/− and Negr1+/+ (wild-type [WT]) C57BL/6 mice (n = 5–14). Intracellular lipid content was measured, and lipid accumulation was visualized by staining tissue cross-sections with lipid-specific stains. Muscle capacity of the WT and Negr1−/− mice was determined by performing a treadmill endurance test, and muscle fiber size was examined. Plasma glucose and insulin levels were measured, and glucose and insulin tolerance tests were performed.
Results
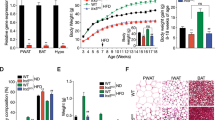
The Negr1−/− mice showed a significant increase in fat mass (~1.5-fold increase in the epididymal white adipose tissue, p = 0.000002), with abnormally enlarged adipose cells, compared with the WT mice. Primary adipocytes of the Negr1−/− mice contained enlarged cytosolic lipid droplets (p = 0.049). Moreover, these mice showed significant hepatic lipid accumulation (~2.3-fold increase, p = 0.043). Although the Negr1−/− mice did not show a significant change in plasma lipoprotein level, they showed a >1.3-fold increase in a serum glucose (p = 0.0002) and insulin (p = 0.016) levels. Moreover, the Negr1−/− mice showed decreased muscle capacity, as indicated by a decrease in muscle mass (p = 0.000003).
Conclusion
These results indicate that NEGR1 deficiency induces abnormal fat deposition in various peripheral cells, especially fat and liver tissue cells, and suggest that NEGR1 is a potential molecular target for designing anti-obesity drugs to regulate body weight both centrally and peripherally.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Funatsu N, Miyata S, Kumanogoh H, Shigeta M, Hamada K, Endo Y, et al. Characterization of a novel rat brain glycosylphosphatidylinositol-anchored protein (Kilon), a member of the IgLON cell adhesion molecule family. J Biol Chem. 1999;274:8224–30.
Hashimoto T, Maekawa S, Miyata S. IgLON cell adhesion molecules regulate synaptogenesis in hippocampal neurons. Cell Biochem Funct. 2009;27:496–8.
Kim H, Hwang JS, Lee B, Hong J, Lee S. Newly Identified Cancer-Associated Role of Human Neuronal Growth Regulator 1 (NEGR1). J Cancer. 2014;5:598–608.
Willer CJ, Speliotes EK, Loos RJ, Li S, Lindgren CM, Heid IM, et al. Six new loci associated with body mass index highlight a neuronal influence on body weight regulation. Nat Genet. 2009;41:25–34.
Wheeler E, Huang N, Bochukova EG, Keogh JM, Lindsay S, Garg S, et al. Genome-wide SNP and CNV analysis identifies common and low-frequency variants associated with severe early-onset obesity. Nat Genet. 2013;45:513–7.
Lee AW, Hengstler H, Schwald K, Berriel-Diaz M, Loreth D, Kirsch M, et al. Functional inactivation of the genome-wide association study obesity gene neuronal growth regulator 1 in mice causes a body mass phenotype. PLoS ONE. 2012;7:e41537.
Boender AJ, van Gestel MA, Garner KM, Luijendijk MC, Adan RA. The obesity-associated gene Negr1 regulates aspects of energy balance in rat hypothalamic areas. Physiological reports. 2014;2:e12083.
Kim H, Chun Y, Che L, Kim J, Lee S, Lee S. The new obesity-associated protein, neuronal growth regulator 1 (NEGR1), is implicated in Niemann-Pick disease Type C (NPC2)-mediated cholesterol trafficking. Biochem Biophys Res Commun. 2017;482:1367–74.
Chun Y, Kim R, Lee S. Centromere Protein (CENP)-W interacts with Heterogeneous Nuclear Ribonucleoprotein (hnRNP) U and may contribute to kinetochore-microtubule attachment in mitotic cells. PLoS ONE. 2016;11:e0149127.
Fisher FM, Kleiner S, Douris N, Fox EC, Mepani RJ, Verdeguer F, et al. FGF21 regulates PGC-1alpha and browning of white adipose tissues in adaptive thermogenesis. Genes Dev. 2012;26:271–81.
Handschin C, Chin S, Li P, Liu F, Maratos-Flier E, Lebrasseur NK, et al. Skeletal muscle fiber-type switching, exercise intolerance, and myopathy in PGC-1alpha muscle-specific knock-out animals. J Biol Chem. 2007;282:30014–21.
Lefterova MI, Zhang Y, Steger DJ, Schupp M, Schug J, Cristancho A, et al. PPARgamma and C/EBP factors orchestrate adipocyte biology via adjacent binding on a genome-wide scale. Genes Dev. 2008;22:2941–52.
Beller M, Thiel K, Thul PJ, Jackle H. Lipid droplets: a dynamic organelle moves into focus. FEBS Lett. 2010;584:2176–82.
Morrison S, McGee SL. 3T3-L1 adipocytes display phenotypic characteristics of multiple adipocyte lineages. Adipocyte. 2015;4:295–302.
Ducharme NA, Bickel PE. Lipid droplets in lipogenesis and lipolysis. Endocrinology. 2008;149:942–9.
Bechmann LP, Hannivoort RA, Gerken G, Hotamisligil GS, Trauner M, Canbay A. The interaction of hepatic lipid and glucose metabolism in liver diseases. J Hepatol. 2012;56:952–64.
Wendel AA, Lewin TM, Coleman RA. Glycerol-3-phosphate acyltransferases: rate limiting enzymes of triacylglycerol biosynthesis. Biochim Biophys Acta. 2009;1791:501–6.
Lee SJ, Kang JH, Iqbal W, Kwon OS. Proteomic analysis of mice fed methionine and choline deficient diet reveals marker proteins associated with steatohepatitis. PLoS ONE. 2015;10:e0120577.
Coleman SK, Rebalka IA, D’Souza DM, Hawke TJ. Skeletal muscle as a therapeutic target for delaying type 1 diabetic complications. World J Diabetes. 2015;6:1323–36.
Sutton AK, Myers MG Jr., Olson DP. The Role of PVH Circuits in Leptin Action and Energy Balance. Annu Rev Physiol. 2016;78:207–21.
Yan X, Wang Z, Schmidt V, Gauert A, Willnow TE, Heinig M, et al. Cadm2 regulates body weight and energy homeostasis in mice. Mol Metab. 2018;8:180–8.
Yang HJ, Xia YY, Wang L, Liu R, Goh KJ, Ju PJ, et al. A novel role for neural cell adhesion molecule in modulating insulin signaling and adipocyte differentiation of mouse mesenchymal stem cells. J Cell Sci. 2011;124(Pt 15):2552–60.
Miyata S, Taguchi K, Maekawa S. Dendrite-associated opioid-binding cell adhesion molecule localizes at neurosecretory granules in the hypothalamic magnocellular neurons. Neuroscience. 2003;122:169–81.
Miyata S, Matsumoto N, Taguchi K, Akagi A, Iino T, Funatsu N, et al. Biochemical and ultrastructural analyses of IgLON cell adhesion molecules, Kilon and OBCAM in the rat brain. Neuroscience. 2003;117:645–58.
Speakman JR. Functional analysis of seven genes linked to body mass index and adiposity by genome-wide association studies: a review. Hum Hered. 2013;75:57–79.
Simon MM, Greenaway S, White JK, Fuchs H, Gailus-Durner V, Wells S, et al. A comparative phenotypic and genomic analysis of C57BL/6J and C57BL/6N mouse strains. Genome Biol. 2013;14:R82.
Fisher-Wellman KH, Ryan TE, Smith CD, Gilliam LA, Lin CT, Reese LR, et al. A direct comparison of metabolic responses to high-fat diet in C57BL/6J and C57BL/6NJ mice. Diabetes. 2016;65:3249–61.
Pol A, Gross SP, Parton RG. Review: biogenesis of the multifunctional lipid droplet: lipids, proteins, and sites. J Cell Biol. 2014;204:635–46.
Wilfling F, Haas JT, Walther TC, Farese RV Jr. Lipid droplet biogenesis. Curr Opin Cell Biol. 2014;29:39–45.
Jacquier N, Choudhary V, Mari M, Toulmay A, Reggiori F, Schneiter R. Lipid droplets are functionally connected to the endoplasmic reticulum in Saccharomyces cerevisiae. J Cell Sci. 2011;124(Pt 14):2424–37.
Mishra S, Khaddaj R, Cottier S, Stradalova V, Jacob C, Schneiter R. Mature lipid droplets are accessible to ER luminal proteins. J Cell Sci. 2016;129:3803–15.
Imamura M, Inoguchi T, Ikuyama S, Taniguchi S, Kobayashi K, Nakashima N, et al. ADRP stimulates lipid accumulation and lipid droplet formation in murine fibroblasts. Am J Physiol Endocrinol Metab. 2002;283:E775–83.
Acknowledgements
This research is supported by the Basic Science Research Program of the Ministry of Education, Science and Technology of Korea (NRF-2016R1A2B4009244).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Joo, Y., Kim, H., Lee, S. et al. Neuronal growth regulator 1-deficient mice show increased adiposity and decreased muscle mass. Int J Obes 43, 1769–1782 (2019). https://doi.org/10.1038/s41366-019-0376-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41366-019-0376-2
This article is cited by
-
Genetics and epigenetics in the obesity phenotyping scenario
Reviews in Endocrine and Metabolic Disorders (2023)
-
Reduced secretion of neuronal growth regulator 1 contributes to impaired adipose-neuronal crosstalk in obesity
Nature Communications (2022)
-
The genetics of obesity: from discovery to biology
Nature Reviews Genetics (2022)
-
Association of genetic variants and behavioral factors with the risk of metabolic syndrome in Pakistanis
Biologia (2022)
-
Shared genetic etiology and causality between body fat percentage and cardiovascular diseases: a large-scale genome-wide cross-trait analysis
BMC Medicine (2021)